Pharmacokinetic Profile and Anti-Adhesive Effect of Oxaliplatin-PLGA Microparticle-Loaded Hydrogels in Rats for Colorectal Cancer Treatment
Abstract
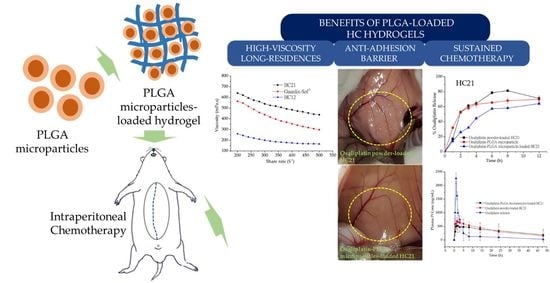
:1. Introduction
2. Materials and Methods
2.1. Materials and Animals
2.2. Preparation of Oxaliplatin-PLGA Microparticles
2.3. Synthesis of HA-CMCNa Cross-Linked Hydrogels
2.4. Preparation of Oxaliplatin-PLGA Microparticle-Loaded Hydrogel
2.5. Characterization of Oxaliplatin-PLGA Microparticles
2.5.1. Morphology
2.5.2. Particle Size Analysis
2.5.3. Rheological Measurements
2.5.4. Encapsulation Efficiency
2.6. In Vitro Oxaliplatin Release from PLGA Microparticles Loaded into Hydrogels
2.7. In Vivo Oxaliplatin Release in SD Rat’s Intraperitoneal Cavity
2.8. High-Performance Liquid Chromatography (HPLC) Analysis
3. Results and Discussion
3.1. Characterization of PLGA Microparticles
3.2. Preparation and Characterization of HC Hydrogels
3.3. In Vitro Oxaliplatin Release Study
3.4. Pharmacokinetics in Rats
3.5. Intraperitoneal Anti-Adhesion Effect
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Verwaal, V.J.; van Ruth, S.; Witkamp, A.; Boot, H.; van Slooten, G.; Zoetmulder, F.A.N. Long-term survival of peritoneal carcinomatosis of colorectal origin. Ann. Surg. Oncol. 2005, 12, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Koppe, M.J.; Boerman, O.C.; Oyen, W.J.G.; Bleichrodt, R.P. Peritoneal carcinomatosis of colorectal origin—Incidence and current treatment strategies. Ann. Surg. 2006, 243, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Verwaal, V.J.; van Ruth, S.; de Bree, E.; van Slooten, G.W.; van Tinteren, H.; Boot, H.; Zoetmulder, F.A.N. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J. Clin. Oncol. 2003, 21, 3737–3743. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.P.; Freeman, M.L. Clinical implications of postsurgical adhesions. Hum. Reprod. Update 2001, 7, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Dizerega, G.S. Contemporary Adhesion Prevention. Fertil. Steril. 1994, 61, 219–235. [Google Scholar] [CrossRef]
- Oh, A. Trends of anti-adhesion adjuvant—Review. Biomater. Res. 2013, 17, 138–145. [Google Scholar]
- Lim, W.Q.; Phua, S.Z.F.; Chen, H.Z.; Zhao, Y.L. An oxaliplatin(iv) prodrug-based supramolecular self-delivery nanocarrier for targeted colorectal cancer treatment. Chem. Commun. 2018, 54, 12762–12765. [Google Scholar] [CrossRef]
- Serrano, D.R.; Hernandez, L.; Fleire, L.; Gonzalez-Alvarez, I.; Montoya, A.; Ballesteros, M.P.; Dea-Ayuela, M.A.; Miro, G.; Bolas-Fernandez, F.; Torrado, J.J. Hemolytic and pharmacokinetic studies of liposomal and particulate amphotericin B formulations. Int. J. Pharm. 2013, 447, 38–46. [Google Scholar] [CrossRef]
- Song, N.; Pogue-Geile, K.L.; Gavin, P.G.; Yothers, G.; Kim, S.R.; Johnson, N.L.; Lipchik, C.; Allegra, C.J.; Petrelli, N.J.; O’Connell, M.J.; et al. Clinical Outcome From Oxaliplatin Treatment in Stage II/III Colon Cancer According to Intrinsic Subtypes Secondary Analysis of NSABP C-O7/NRG Oncology Randomized Clinical Trial. JAMA Oncol. 2016, 2, 1162–1169. [Google Scholar] [CrossRef]
- Piche, N.; Leblond, F.A.; Sideris, L.; Pichette, V.; Drolet, P.; Fortier, L.P.; Mitchell, A.; Dube, P. Rationale for Heating Oxaliplatin for the Intraperitoneal Treatment of Peritoneal Carcinomatosis A Study of the Effect of Heat on Intraperitoneal Oxaliplatin Using a Murine Model. Ann. Surg. 2011, 254, 138–144. [Google Scholar] [CrossRef]
- Elias, D.; Raynard, B.; Farkhondeh, F.; Goere, D.; Rouquie, D.; Ciuchendea, R.; Pocard, M.; Ducreux, M. Peritoneal carcinomatosis of colorectal origin—Long-term results of intraperitoneal chemohyperthermia with oxaliplatin following complete cytoreductive surgery. Gastroen. Clin. Biol. 2006, 30, 1200–1204. [Google Scholar] [CrossRef]
- Makkouk, A.; Joshi, V.B.; Wongrakpanich, A.; Lemke, C.D.; Gross, B.P.; Salem, A.K.; Weiner, G.J. Biodegradable Microparticles Loaded with Doxorubicin and CpG ODN for In Situ Immunization Against Cancer. AAPS J. 2015, 17, 184–193. [Google Scholar] [CrossRef]
- Ramasamy, T.; Ruttala, H.B.; Gupta, B.; Poudel, B.K.; Choi, H.G.; Yong, C.S.; Kim, J.O. Smart chemistry-based nanosized drug delivery systems for systemic applications: A comprehensive review. J. Control. Release 2017, 258, 226–253. [Google Scholar] [CrossRef]
- Blasi, P. Poly(lactic acid)/poly(lactic-co-glycolic acid)-based microparticles: An overview. J. Pharm. Investig. 2019, 49, 337–346. [Google Scholar] [CrossRef]
- Taghizadehghalehjoughi, A.; Hacimuftuoglu, A.; Cetin, M.; Ugur, A.B.; Galateanu, B.; Mezhuev, Y.; Okkay, U.; Taspinar, N.; Taspinar, M.; Uyanik, A.; et al. Effect of metformin/irinotecan-loaded poly-lactic-co-glycolic acid nanoparticles on glioblastoma: In vitro and in vivo studies. Nanomedicine 2018, 13, 1595–1606. [Google Scholar] [CrossRef]
- Palileo, A.; Munoz-Sagastibelza, M.; Martello-Rooney, L. Treatment with paclitaxel causes upregulation in resistance protein tubulin beta III in a type 2 human endometrial cancer cell line. Gynecol. Oncol. 2019, 154, e15. [Google Scholar] [CrossRef]
- Dwivedi, P.; Han, S.Y.; Mangrio, F.; Fan, R.; Dwivedi, M.; Zhu, Z.A.; Huang, F.S.; Wu, Q.; Khatik, R.; Cohn, D.E.; et al. Engineered multifunctional biodegradable hybrid microparticles for paclitaxel delivery in cancer therapy. Mater. Sci. Eng. C-Mater. 2019, 102, 113–123. [Google Scholar] [CrossRef]
- Lee, J.E.; Abuzar, S.M.; Seo, Y.; Han, H.; Jeon, Y.; Park, E.J.; Baik, S.H.; Hwang, S.-J. Oxaliplatin-loaded chemically cross-linked hydrogels for prevention of postoperative abdominal adhesion and colorectal cancer therapy. Int. J. Pharm. 2019, 565, 50–58. [Google Scholar] [CrossRef]
- Naghizadeh, Z.; Karkhaneh, A.; Khojasteh, A. Simultaneous release of melatonin and methylprednisolone from an injectable in situ self-crosslinked hydrogel/microparticle system for cartilage tissue engineering. J. Biomed. Mater. Res. A 2018, 106, 1932–1940. [Google Scholar] [CrossRef]
- Khaing, Z.Z.; Agrawal, N.K.; Park, J.H.; Xin, S.J.; Plumton, G.C.; Lee, K.H.; Huang, Y.J.; Niemerski, A.L.; Schmidt, C.E.; Grau, J.W. Localized and sustained release of brain-derived neurotrophic factor from injectable hydrogel/microparticle composites fosters spinal learning after spinal cord injury. J. Mater. Chem. B 2016, 4, 7560–7571. [Google Scholar] [CrossRef]
- Jain, R.A. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials 2000, 21, 2475–2490. [Google Scholar] [CrossRef]
- Liu, L.; Liu, D.R.; Wang, M.; Du, G.C.; Chen, J. Preparation and characterization of sponge-like composites by cross-linking hyaluronic acid and carboxymethylcellulose sodium with adipic dihydrazide. Eur. Polym. J. 2007, 43, 2672–2681. [Google Scholar] [CrossRef]
- Marshall, C.D.; Hu, M.S.; Leavitt, T.; Barnes, L.A.; Cheung, A.T.; Malhotra, S.; Lorenz, H.P.; Longaker, M.T. Creation of Abdominal Adhesions in Mice. J. Vis. Exp. 2016, 144. [Google Scholar] [CrossRef]
- Graham, M.A.; Lockwood, G.F.; Greenslade, D.; Brienza, S.; Bayssas, M.; Gamelin, E. Clinical pharmacokinetics of oxaliplatin: A critical review. Clin. Cancer Res. 2000, 6, 1205–1218. [Google Scholar]
- Milas, M.; Rinaudo, M.; Roure, I.; Al-Assaf, S.; Phillips, G.O.; Williams, P.A. Comparative rheological behavior of hyaluronan from bacterial and animal sources with cross-linked hyaluronan (hylan) in aqueous solution. Biopolymers 2001, 59, 191–204. [Google Scholar] [CrossRef]
- Bittner, B.; Witt, C.; Mader, K.; Kissel, T. Degradation and protein release properties of microspheres prepared from biodegradable poly(lactide-co-glycolide) and ABA triblock copolymers: Influence of buffer media on polymer erosion and bovine serum albumin release. J. Control. Release 1999, 60, 297–309. [Google Scholar] [CrossRef]
- Rehman, F.; Volpe, P.L.O.; Airoldi, C. The applicability of ordered mesoporous SBA-15 and its hydrophobic glutaraldehyde-bridge derivative to improve ibuprofen-loading in releasing system. Colloid Surf. B 2014, 119, 82–89. [Google Scholar] [CrossRef]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Chen, C.H.; Chen, S.H.; Mao, S.H.; Tsai, M.J.; Chou, P.Y.; Liao, C.H.; Chen, J.P. Injectable thermosensitive hydrogel containing hyaluronic acid and chitosan as a barrier for prevention of postoperative peritoneal adhesion. Carbohydr. Polym. 2017, 173, 721–731. [Google Scholar] [CrossRef]
- Kasturi, S.P.; Qin, H.; Thomson, K.S.; El-Bereir, S.; Cha, S.C.; Neelapu, S.; Kwak, L.W.; Roy, K. Prophylactic anti-tumor effects in a B cell lymphoma model with DNA vaccines delivered on polyethylenimine (PEI) functionalized PLGA microparticles. J. Control. Release 2006, 113, 261–270. [Google Scholar] [CrossRef]






| Particles | Method | Weight Ratio | Particle Size (nm) (Mean ± SD) | Encapsulation Efficiency (%) (Mean ± SD) | |||
|---|---|---|---|---|---|---|---|
| Oxaliplatin | PLGA 502 H | HA | CMCNa | ||||
| Oxaliplatin-PLGA Microparticles | Double Emulsion | 1 | 50 | - | - | 1100.4 ± 257.7 | 77.9 ± 2.8 |
| HC12 Hydrogel | Synthesis by cross-linking reaction | - | - | 1 | 2 | - | - |
| HC21 Hydrogel | - | - | 2 | 1 | - | - | |
| Equations | Loaded in HC12 Hydrogel | Loaded in Guardix-Sol® Hydrogel | Loaded in HC21 Hydrogel | PLGA Microparticle | |||
|---|---|---|---|---|---|---|---|
| Oxaliplatin Powder | PLGA Microparticle | Oxaliplatin Powder | PLGA Microparticle | Oxaliplatin Powder | PLGA Microparticle | ||
| a Higuchi model: kH (% h1/2) | 28.5970 | 18.9686 | 22.1635 | 17.5626 | 24.3401 | 20.6559 | 20.0882 |
| R2 | 0.6738 | 0.9611 | 0.7431 | 0.9783 | 0.7746 | 0.9485 | 0.8026 |
| b First—Order model: k1 (h−1) | 0.0302 | 0.0421 | 0.0263 | 0.0469 | 0.0379 | 0.0522 | 0.0219 |
| R2 | 0.1639 | 0.6646 | 0.2327 | 0.7224 | 0.2640 | 0.6451 | 0.4145 |
| c Korsmeyer—Peppas model: kKP | 0.4517 | 0.5033 | 0.3750 | 0.5475 | 0.5352 | 0.6308 | 0.2884 |
| R2 | 0.5495 | 0.9342 | 0.6099 | 0.9554 | 0.6512 | 0.9366 | 0.7767 |
| Formulations | Pharmacokinetic Parameters | |||
|---|---|---|---|---|
| Cmax (ng/mL) | Tmax (h) | AUC0–48h (ng·h/mL) | MRT (h) | |
| Oxaliplatin solution | 2265.28 ± 192.51 | 1 | 8181.51 ± 176.89 | 4.36 ± 0.65 |
| Oxaliplatin powder loaded HC21 | 690.63 ± 140.54 | 1.5 | 16571.37 ± 139.13 | 10.29 ± 0.33 |
| Oxaliplatin-PLGA microparticle loaded HC21 | 528.75 ± 144.50 | 1.5 | 16012.12 ± 188.75 | 12.02 ± 0.41 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abuzar, S.M.; Ahn, J.-H.; Park, K.S.; Park, E.J.; Baik, S.H.; Hwang, S.-J. Pharmacokinetic Profile and Anti-Adhesive Effect of Oxaliplatin-PLGA Microparticle-Loaded Hydrogels in Rats for Colorectal Cancer Treatment. Pharmaceutics 2019, 11, 392. https://doi.org/10.3390/pharmaceutics11080392
Abuzar SM, Ahn J-H, Park KS, Park EJ, Baik SH, Hwang S-J. Pharmacokinetic Profile and Anti-Adhesive Effect of Oxaliplatin-PLGA Microparticle-Loaded Hydrogels in Rats for Colorectal Cancer Treatment. Pharmaceutics. 2019; 11(8):392. https://doi.org/10.3390/pharmaceutics11080392
Chicago/Turabian StyleAbuzar, Sharif Md, Jun-Hyun Ahn, Kyung Su Park, Eun Jung Park, Seung Hyuk Baik, and Sung-Joo Hwang. 2019. "Pharmacokinetic Profile and Anti-Adhesive Effect of Oxaliplatin-PLGA Microparticle-Loaded Hydrogels in Rats for Colorectal Cancer Treatment" Pharmaceutics 11, no. 8: 392. https://doi.org/10.3390/pharmaceutics11080392