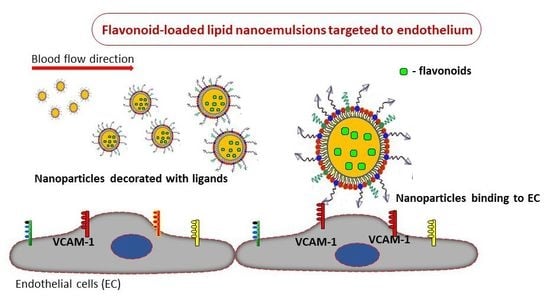
Functional Role of VCAM-1 Targeted Flavonoid-Loaded Lipid Nanoemulsions in Reducing Endothelium Inflammation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of Non-Targeted Flavonoid-Loaded Nanoemulsions
2.3. Preparation of VCAM-1 Targeted Flavonoid-Loaded Nanoemulsions
2.4. Characterization of Flavonoid-Loaded Lipid Nanoemulsions
2.4.1. Size and Zeta Potential
2.4.2. Determination of the Amount of Peptide Coupled to LN Surface
2.4.3. Flavonoid Entrapment into Lipid Nanoemulsions
2.4.4. In Vitro Release of Flavonoid Incorporated in Nanoemulsions
2.5. Cell Culture
2.6. Viability Assay
2.7. Uptake of Lipid Nanoemulsions by Endothelial Cells
2.8. Monocyte Adhesion Assay
2.9. Monocyte Transmigration Assay
2.10. Immunoblotting Assay
2.11. Quantitative RT-PCR
2.12. Hemocompatibility Test
2.13. Statistical Analysis
3. Results
3.1. Preparation and Physicochemical Characterization of Flavonoid-Loaded Nanoemulsions
3.1.1. Size and Zeta Potential
3.1.2. Flavonoid-Encapsulation Efficiency
3.1.3. In Vitro Flavonoid Release
3.1.4. In Vitro Cytotoxicity of Flavonoid-Loaded LNs Assayed on EA.hy926 Cells
3.2. VCAM-1 Targeted LNs Are Taken up at a Higher Extent by TNF-α Activated EA.hy926 Cells as Compared with Non-Activated Cells
3.3. Flavonoid-Loaded LNs Have a Functional Role in Inhibiting Monocyte Adhesion and Transmigration to/through Activated EA.hy926 Cells
3.4. Flavonoid-Loaded LNs Reduce the Expression Level of Pro-Inflammatory Molecules in TNF-α Activated EA.hy926 Cells
3.5. Hemocompatibility of Flavonoid-Loaded LNs
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mulvihill, E.E.; Burke, A.C.; Huff, M.W. Citrus Flavonoids as Regulators of Lipoprotein Metabolism and Atherosclerosis. Annu. Rev. Nutr. 2016, 36, 275–299. [Google Scholar] [CrossRef] [PubMed]
- Testai, L.; Calderone, V. Nutraceutical Value of Citrus Flavanones and Their Implications in Cardiovascular Disease. Nutrients 2017, 9, 502. [Google Scholar] [CrossRef] [PubMed]
- Chanet, A.; Milenkovic, D.; Deval, C.; Potier, M.; Constans, J.; Mazur, A.; Bennetau-Pelissero, C.; Morand, C.; Bérard, A.M. Naringin, the major grapefruit flavonoid, specifically affects atherosclerosis development in diet-induced hypercholesterolemia in mice. J. Nutr. Biochem. 2012, 23, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Chanet, A.; Milenkovic, D.; Claude, S.; Maier, J.A.; Kamran, K.M.; Rakotomanomana, N.; Shinkaruk, S.; Bérard, A.M.; Bennetau-Pelissero, C.; Mazur, A.; et al. Flavanone metabolites decrease monocyte adhesion to TNF-α-activated endothelial cells by modulating expression of atherosclerosis-related genes. Br. J. Nutr. 2013, 110, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Jeong, T.S.; Choi, Y.K.; Hyun, B.H.; Oh, G.T.; Kim, E.H.; Kim, J.R.; Han, J.I.; Bok, S.H. Anti-atherogenic effect of citrus flavonoids, naringin and naringenin, associated with hepatic ACAT and aortic VCAM-1 and MCP-1 in high cholesterol-fed rabbits. Biochem. Biophys. Res. Commun. 2001, 284, 681–688. [Google Scholar] [CrossRef]
- Hsueh, T.P.; Sheen, J.M.; Pang, J.H.; Bi, K.W.; Huang, C.C.; Wu, H.T.; Huang, S.T. The Anti-Atherosclerotic Effect of Naringin Is Associated with Reduced Expressions of Cell Adhesion Molecules and Chemokines through NF-κB Pathway. Molecules 2016, 21, 195. [Google Scholar] [CrossRef] [PubMed]
- DrugBank. Available online: www.drugbank.ca (accessed on 26 July 2019).
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [Green Version]
- Erlund, I.; Silaste, M.L.; Alfthan, G.; Rantala, M.; Kesäniemi, Y.A.; Aro, A. Plasma concentrations of the flavonoids hesperetin, naringenin and quercetin in human subjects following their habitual diets, and diets high or low in fruit and vegetables. Eur. J. Clin. Nutr. 2002, 56, 891–898. [Google Scholar] [CrossRef] [Green Version]
- Erlund, I.; Meririnne, E.; Alfthan, G.; Aro, A. Plasma kinetics and urinary excretion of the flavanones naringenin and hesperetin in humans after ingestion of orange juice and grapefruit juice. J. Nutr. 2001, 131, 235–241. [Google Scholar] [CrossRef]
- Shulman, M.; Cohen, M.; Soto-Gutierrez, A.; Yagi, H.; Wang, H.; Goldwasser, J.; Lee-Parsons, C.W.; Benny-Ratsaby, O.; Yarmush, M.L.; Nahmias, Y. Enhancement of naringenin bioavailability by complexation with hydroxypropyl-β-cyclodextrin. [corrected]. PLoS ONE 2011, 6, e18033. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Firempong, C.K.; Zhang, H.; Wang, M.; Zhang, Y.; Zhu, Y.; Yu, J.; Xu, X. Enhanced Solubility and Bioavailability of Naringenin via Liposomal Nanoformulation: Preparation and In Vitro and In Vivo Evaluations. AAPS Pharm. Sci. Tech. 2017, 18, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Mukhopadhyay, P.; Kundu, P.P.; Chakraborti, A.S. Alginate coated chitosan core-shell nanoparticles for efficient oral delivery of naringenin in diabetic animals-An in vitro and in vivo approach. Carbohydr. Polym. 2017, 170, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Simion, V.; Stan, D.; Constantinescu, C.A.; Deleanu, M.; Dragan, E.; Tucureanu, M.M.; Gan, A.M.; Butoi, E.; Constantin, A.; Manduteanu, I.; et al. Conjugation of curcumin-loaded lipid nanoemulsions with cell-penetrating peptides increases their cellular uptake and enhances the anti-inflammatory effects in endothelial cells. J. Pharm. Pharmacol. 2016, 68, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.K. Linking endothelial dysfunction with endothelial cell activation. J. Clin. Investig. 2013, 123, 540–541. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, M.P.; Gimbrone, M.A., Jr. Inducible endothelial functions in inflammation and coagulation. Semin. Thromb. Hemost. 1987, 13, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Khodabandehlou, K.; Masehi-Lano, J.J.; Poon, C.; Wang, J.; Chung, E.J. Targeting cell adhesion molecules with nanoparticles using in vivo and flow-based in vitro models of atherosclerosis. Exp. Biol. Med. 2017, 242, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Calin, M.; Manduteanu, I. Emerging Nanocarriers-based Approaches to Diagnose and Reduce Vascular Inflammation in Atherosclerosis. Curr. Med. Chem. 2017, 24, 550–567. [Google Scholar] [CrossRef] [PubMed]
- Simion, V.; Constantinescu, C.A.; Stan, D.; Deleanu, M.; Tucureanu, M.M.; Butoi, E.; Manduteanu, I.; Simionescu, M.; Calin, M. P-Selectin Targeted Dexamethasone-Loaded Lipid Nanoemulsions: A Novel Therapy to Reduce Vascular Inflammation. Mediat. Inflamm. 2016, 2016, 1625149. [Google Scholar] [CrossRef]
- Constantinescu, C.A.; Fuior, E.V.; Rebleanu, D.; Deleanu, M.; Simion, V.; Voicu, G.; Escriou, V.; Manduteanu, I.; Simionescu, M.; Calin, M. Targeted Transfection Using PEGylated Cationic Liposomes Directed Towards P-Selectin Increases siRNA Delivery into Activated Endothelial Cells. Pharmaceutics 2019, 11, 47. [Google Scholar] [CrossRef]
- Calin, M.; Stan, D.; Schlesinger, M.; Simion, V.; Deleanu, M.; Constantinescu, C.A.; Gan, A.M.; Pirvulescu, M.M.; Butoi, E.; Manduteanu, I.; et al. VCAM-1 directed target-sensitive liposomes carrying CCR2 antagonists bind to activated endothelium and reduce adhesion and transmigration of monocytes. Eur. J. Pharm. Biopharm. 2015, 89, 18–29. [Google Scholar] [CrossRef]
- Roblek, M.; Calin, M.; Schlesinger, M.; Stan, D.; Zeisig, R.; Simionescu, M.; Bendas, G.; Borsig, L. Targeted delivery of CCR2 antagonist to activated pulmonary endothelium prevents metastasis. J. Control. Release 2015, 220, 341–347. [Google Scholar] [CrossRef] [Green Version]
- Kelly, K.A.; Nahrendorf, M.; Yu, A.M.; Reynolds, F.; Weissleder, R. In vivo phage display selection yields atherosclerotic plaque targeted peptides for imaging. Mol. Imaging Biol. 2006, 8, 201–207. [Google Scholar] [CrossRef]
- Manduteanu, I.; Voinea, M.; Antohe, F.; Dragomir, E.; Capraru, M.; Radulescu, L.; Simionescu, M. Effect of enoxaparin on high glucose-induced activation of endothelial cells. Eur. J. Pharmacol. 2003, 477, 269–276. [Google Scholar] [CrossRef]
- Sheffield, J.B.; Graff, D.; Li, H.P. A solid-phase method for the quantitation of protein in the presence of sodium dodecyl sulfate and other interfering substances. Anal. Biochem. 1987, 166, 49–54. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Uritu, C.M.; Calin, M.; Maier, S.S.; Cojocaru, C.; Nicolescu, A.; Peptanariu, D.; Constantinescu, C.A.; Stan, D.; Barboiu, M.; Pinteala, M. Flexible cyclic siloxane core enhances the transfection efficiency of polyethylenimine-based non-viral gene vectors. J. Mater. Chem. B 2015, 3, 8250–8267. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta Potential Measurement in Scott. In Characterization of Nanoparticles Intended for Drug Delivery, Methods in Molecular Biology; McNeil, E., Ed.; Springer: Berlin, Germany, 2011; Volume 697. [Google Scholar] [CrossRef]
- Kozlowski, L.P. IPC—Isoelectric Point Calculator. Biol. Direct 2016, 11, 55. [Google Scholar] [CrossRef]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The Therapeutic Potential of Naringenin: A Review of Clinical Trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Manduteanu, I.; Dragomir, E.; Voinea, M.; Capraru, M.; Simionescu, M. Enoxaparin reduces H2O2-induced activation of human endothelial cells by a mechanism involving cell adhesion molecules and nuclear transcription factors. Pharmacology 2007, 79, 154–162. [Google Scholar] [CrossRef]
- Weber, M.; Steinle, H.; Golombek, S.; Hann, L.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Blood-Contacting Biomaterials: In Vitro Evaluation of the Hemocompatibility. Front. Bioeng. Biotechnol. 2018, 6, 99. [Google Scholar] [CrossRef]
- Zeng, W.; Jin, L.; Zhang, F.; Zhang, C.; Liang, W. Naringenin as a potential immunomodulator in therapeutics. Pharmacol. Res. 2018, 135, 122–126. [Google Scholar] [CrossRef]
- Tejada, S.; Pinya, S.; Martorell, M.; Capó, X.; Tur, J.A.; Pons, A.; Sureda, A. Potential Anti-inflammatory Effects of Hesperidin from the Genus Citrus. Curr. Med. Chem. 2018, 25, 4929–4945. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef]
- Rani, N.; Bharti, S.; Krishnamurthy, B.; Bhatia, J.; Sharma, C.; Kamal, M.A.; Ojha, S.; Arya, D.S. Pharmacological Properties and Therapeutic Potential of Naringenin: A Citrus Flavonoid of Pharmaceutical Promise. Curr. Pharm. Des. 2016, 22, 4341–4359. [Google Scholar] [CrossRef]
- Ren, H.; Hao, J.; Liu, T.; Zhang, D.; Lv, H.; Song, E.; Zhu, C. Hesperetin Suppresses Inflammatory Responses in Lipopolysaccharide-Induced RAW 264.7 Cells via the Inhibition of NF-κB and Activation of Nrf2/HO-1 Pathways. Inflammation 2016, 39, 964–973. [Google Scholar] [CrossRef]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models. Phytother. Res. 2015, 29, 323–331. [Google Scholar] [CrossRef]
- Mestas, J.; Ley, K. Monocyte-endothelial cell interactions in the development of atherosclerosis. Trends Cardiovasc. Med. 2008, 18, 228–232. [Google Scholar] [CrossRef]
- Voinea, M.; Manduteanu, I.; Dragomir, E.; Capraru, M.; Simionescu, M. Immunoliposomes directed toward VCAM-1 interact specifically with activated endothelial cells--a potential tool for specific drug delivery. Pharm. Res. 2005, 22, 1906–1917. [Google Scholar] [CrossRef]
- Ramteke, K.H.; Dighe, P.A.; Kharat, A.R.; Patil, S.V. Mathematical Models of Drug Dissolution: A Review. Sch. Acad. J. Pharm. 2014, 3, 388–396. [Google Scholar]
- Chrastina, A.; Baron, V.T.; Abedinpour, P.; Rondeau, G.; Welsh, J.; Borgström, P. Plumbagin-Loaded Nanoemulsion Drug Delivery Formulation and Evaluation ofAntiproliferative Effect on Prostate Cancer Cells. Biomed. Res. Int. 2018, 2018, 9035452. [Google Scholar] [CrossRef]
- Lin, J.; Kakkar, V.; Lu, X. Impact of MCP-1 in atherosclerosis. Curr. Pharm. Des. 2014, 20, 4580–4588. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1(MCP-1): An overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef]
- Kempe, S.; Kestler, H.; Lasar, A.; Wirth, T. NF-kappaB controls the global pro-inflammatory response in endothelial cells: Evidence for the regulation of a pro-atherogenic program. Nucleic Acids Res. 2005, 33, 5308–5319. [Google Scholar] [CrossRef]
- Joshi, H.; Hegde, A.R.; Shetty, P.K.; Gollavilli, H.; Managuli, R.S.; Kalthur, G.; Mutalik, S. Sunscreen creams containing naringenin nanoparticles: Formulation development and in vitro and in vivo evaluations. Photodermatol. Photoimmunol. Photomed. 2018, 34, 69–81. [Google Scholar] [CrossRef]
- Sandhu, P.S.; Kumar, R.; Beg, S.; Jain, S.; Kushwah, V.; Katare, O.P.; Singh, B. Natural lipids enriched self-nano-emulsifying systems for effective co-delivery of tamoxifen and naringenin: Systematic approach for improved breast cancer therapeutics. Nanomedicine 2017, 13, 1703–1713. [Google Scholar] [CrossRef]
- Parashar, P.; Rathor, M.; Dwivedi, M.; Saraf, S.A. Hyaluronic Acid Decorated Naringenin Nanoparticles: Appraisal of Chemopreventive and Curative Potential for Lung Cancer. Pharmaceutics 2018, 10, 33. [Google Scholar] [CrossRef]







| Flavanone | Naringenin | Hesperetin |
|---|---|---|
| Structure |  |  |
| IUPAC Name | 5,7-Dihydroxy-2-(4-hydroxyphenyl) chroman-4-one | (S)-2,3-Dihydro-5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-4H-1-benzopyran-4-one |
| CAS ID | 480-41-1 | 520-33-2 |
| Main Glycosides | naringin(naringenin-7-neohesperidoside) narirutin (naringenin-7-rutinoside) | hesperidin (hesperetin 7-rutinoside) neohesperidin (hesperetin 7-neohesperidoside) |
| Main Citrus Source | grapefruit | orange |
| A. Storage at 4 °C | Time: 0 | 2 weeks | 4 weeks | 6 weeks | 3 months | |||
| Nar/LN | Size (nm) | 208.2 ± 1.6 | 205.6 ± 0.9 | 203.9 ± 1.1 | 205.7 ± 0.4 | 204.3 ± 0.3 | ||
| PDI | 0.19 ± 0.015 | 0.181± 0.014 | 0.181± 0.019 | 0.186 ± 0.02 | 0.214 ± 0.006 | |||
| Zeta potential (mV) | −35.3 ± 5.1 | −35.5 ± 0.3 | −32.6 ± 3.1 | −33.8 ± 0.5 | −38.1± 3.6 | |||
| V-Nar/LN | Size (nm) | 210.2 ± 1.6 | 214.4 ± 2.2 | 214.3 ± 0.9 | 217.1 ± 3.2 | 227.1 ± 3.3 | ||
| PDI | 0.200 ± 0.01 | 0.205 ± 0.037 | 0.183 ± 0.03 | 0.189 ± 0.02 | 0.223± 0.012 | |||
| Zeta potential (mV) | −52.2 ± 2.3 | −53.5 ± 2.06 | −55.6 ± 1.7 | −54.3 ± 1.4 | −55.1 ± 1.1 | |||
| B. Storage at 37°C | Time: 0 | 1 day | 3 days | 7 days | ||||
| Nar/LN | Size (nm) | 206.2 ± 1.8 | 206.0 ± 1.3 | 197.9 ± 2.5 | 202.4 ± 0.2 | |||
| PDI | 0.217 ± 0.008 | 0.199 ± 0.01 | 0.219 ± 0.009 | 0.174 ± 0.003 | ||||
| Zeta potential (mV) | −29.1 ± 1.6 | −28.7 ± 1.1 | −27.6 ± 1.3 | −25.6 ± 1.8 | ||||
| V-Nar/LN | Size (nm) | 214.0 ± 1.3 | 204.3 ± 1.3 | 205.8 ± 1.015 | 206.0 ± 0.7 | |||
| PDI | 0.224 ± 0.009 | 0.219 ± 0.009 | 0.233 ± 0.005 | 0.22 ± 0.004 | ||||
| Zeta potential (mV) | −48.7 ± 1.1 | −49.5 ± 2.7 | −49.1 ± 0.6 | −52.0 ± 0.4 | ||||
| Parameter | Free Nar | Nar/LNs | V-Nar/LNs | Free Hesp | Hesp/LNs | V-Hesp/LNs |
|---|---|---|---|---|---|---|
| Plateau y0 | 88.28 | 54.46 | 51.98 | 82.67 | 39.74 | 39.08 |
| Half-time | 0.8301 | 0.9046 | 0.9802 | 0.7785 | 0.9957 | 0.6757 |
| R2 | 0.9962 | 0.9985 | 0.9954 | 0.9959 | 0.9893 | 0.9972 |
| Sum of squares | 24.54 | 3.795 | 10.52 | 23.18 | 13.96 | 3.463 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuior, E.V.; Deleanu, M.; Constantinescu, C.A.; Rebleanu, D.; Voicu, G.; Simionescu, M.; Calin, M. Functional Role of VCAM-1 Targeted Flavonoid-Loaded Lipid Nanoemulsions in Reducing Endothelium Inflammation. Pharmaceutics 2019, 11, 391. https://doi.org/10.3390/pharmaceutics11080391
Fuior EV, Deleanu M, Constantinescu CA, Rebleanu D, Voicu G, Simionescu M, Calin M. Functional Role of VCAM-1 Targeted Flavonoid-Loaded Lipid Nanoemulsions in Reducing Endothelium Inflammation. Pharmaceutics. 2019; 11(8):391. https://doi.org/10.3390/pharmaceutics11080391
Chicago/Turabian StyleFuior, Elena Valeria, Mariana Deleanu, Cristina Ana Constantinescu, Daniela Rebleanu, Geanina Voicu, Maya Simionescu, and Manuela Calin. 2019. "Functional Role of VCAM-1 Targeted Flavonoid-Loaded Lipid Nanoemulsions in Reducing Endothelium Inflammation" Pharmaceutics 11, no. 8: 391. https://doi.org/10.3390/pharmaceutics11080391