Hyaluronan Graft Copolymers Bearing Fatty-Acid Residues as Self-Assembling Nanoparticles for Olanzapine Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization
N-(17-Azido-3,6,9,12,15-pentaoxaheptadecyl)oleamide (4a)
N-(17-Azido-3,6,9,12,15-pentaoxaheptadecyl)stearamide (4b)
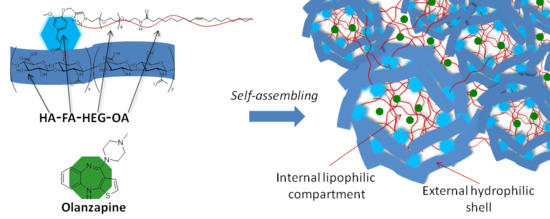
Preparation of HA-FA-HEG-OA
Preparation of HA-FA-HEG-SA
(E)-Methyl 3-[3-methoxy-4-[[1-[(Z)-19-oxo-3,6,9,12,15-pentaoxa-18-azahexatriacont-27-en-1-yl]-1H-1,2,3-triazol-4-yl]methoxy]phenyl]acrylate (FE-HEG-OA)
(E)-3-[3-Methoxy-4-[[1-[(Z)-19-oxo-3,6,9,12,15-pentaoxa-18-azahexatriacont-27-en-1-yl]-1H-1,2,3-triazol-4-yl]methoxy]phenyl]acrylic acid (FA-HEG-OA)
2.2. Particle Size Analysis and Zeta Potential
2.3. Critical Aggregation Concentration
2.4. Transmission Electron Microscopy (TEM) Analysis
2.5. Rheological Analysis
2.6. Preparation of Self-Assembling Nanoparticles
2.7. Drug Content and Encapsulation Efficiency
2.8. Drug Release Studies
2.9. Cytotoxicity Assay
2.10. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of HA-FA-HEG-OA and HA-FA-HEG-SA Materials
3.2. Structure of HA-FA-HEG-OA and HA-FA-HEG-SA Materials
3.3. Self-Assembling Properties
3.4. Olanzapine Loading and Release
3.5. In Vitro Biological Evaluations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cohen, M.; Joester, D.; Geiger, B.; Addadi, L. Spatial and Temporal Sequence of Events in Cell Adhesion: From Molecular Recognition to Focal Adhesion Assembly. ChemBioChem 2004, 5, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Goltsche, K.; Cheng, L.; Xie, F.; Meng, F.; Deng, C.; Zhong, Z.; Haag, R. Hyaluronic acid-shelled acid-activatable paclitaxel prodrug micelles effectively target and treat CD44-overexpressing human breast tumor xenografts in vivo. Biomaterials 2016, 84, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Dosio, F.; Arpicco, S.; Stella, B.; Fattal, E. Hyaluronic acid for anticancer drug and nucleic acid delivery. Adv. Drug Deliv. Rev. 2016, 97, 204–236. [Google Scholar] [CrossRef] [PubMed]
- Orian-Rousseau, V. CD44, a therapeutic target for metastasising tumours. Eur. J. Cancer 2010, 46, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, A.; Grisci, G.; Paolino, M.; Giuliani, G.; Donati, A.; Mendichi, R.; Artusi, R.; Demiranda, M.; Zanardi, A.; Giorgi, G.; et al. Hyaluronan derivatives bearing variable densities of ferulic acid residues. J. Mater. Chem. B 2014, 2, 4489–4499. [Google Scholar] [CrossRef]
- Valacchi, G.; Grisci, G.; Sticozzi, C.; Lim, Y.; Paolino, M.; Giuliani, G.; Mendichi, R.; Belmonte, G.; Artusi, R.; Zanardi, A.; et al. Wound healing properties of hyaluronan derivatives bearing ferulate residues. J. Mater. Chem. B 2015, 3, 7037–7045. [Google Scholar] [CrossRef]
- Paolino, M.; Komber, H.; Mennuni, L.; Caselli, G.; Appelhans, D.; Voit, B.; Cappelli, A. Supramolecular glycodendrimer-based hybrid drugs. Biomacromolecules 2014, 15, 3985–3993. [Google Scholar] [CrossRef]
- Cappelli, A.; Grisci, G.; Paolino, M.; Castriconi, F.; Giuliani, G.; Donati, A.; Lamponi, S.; Mendichi, R.; Boccia, A.C.; Samperi, F.; et al. Combining spontaneous polymerization and click reactions for the synthesis of polymer brushes: A “grafting onto” approach. Chem. A Eur. J. 2013, 19, 9710–9721. [Google Scholar] [CrossRef]
- Paolino, M.; Mennuni, L.; Giuliani, G.; Anzini, M.; Lanza, M.; Caselli, G.; Galimberti, C.; Menziani, M.C.; Donati, A.; Cappelli, A. Dendrimeric tetravalent ligands for the serotonin-gated ion channel. Chem. Commun. (Camb). 2014, 50, 8582–8585. [Google Scholar] [CrossRef]
- Pagano, K.; Paolino, M.; Fusi, S.; Zanirato, V.; Trapella, C.; Giuliani, G.; Cappelli, A.; Zanzoni, S.; Molinari, H.; Ragona, L.; et al. Bile Acid Binding Protein Functionalization Leads to a Fully Synthetic Rhodopsin Mimic. J. Phys. Chem. Lett. 2019, 10, 2235–2243. [Google Scholar] [CrossRef]
- Cappelli, A.; Paolino, M.; Reale, A.; Razzano, V.; Grisci, G.; Giuliani, G.; Donati, A.; Bonechi, C.; Lamponi, S.; Mendichi, R.; et al. Hyaluronan-based graft copolymers bearing aggregation-induced emission fluorogens. RSC Adv. 2018, 8, 5864–5881. [Google Scholar] [CrossRef] [Green Version]
- Atrei, A.; Innocenti, C.; Lamponi, S.; Paesano, S.; Leone, G.; Reale, A.; Paolino, M.; Cappelli, A. Covalent hyaluronic-based coating of magnetite nanoparticles: Preparation, physicochemical and biological characterization. Mater. Sci. Eng. C 2020, 107, 110271. [Google Scholar] [CrossRef] [PubMed]
- Paolino, M.; Grisci, G.; Reale, A.; Razzano, V.; Giuliani, G.; Donati, A.; Mendichi, R.; Piovani, D.; Boccia, A.C.; Grillo, A.; et al. Structural manipulation of the conjugated phenyl moiety in 3-phenylbenzofulvene monomers: Effects on spontaneous polymerization. Polymers (Basel). 2018, 10, 752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cappelli, A.; Razzano, V.; Paolino, M.; Grisci, G.; Giuliani, G.; Donati, A.; Mendichi, R.; Samperi, F.; Battiato, S.; Boccia, A.C.; et al. Bithiophene-based polybenzofulvene derivatives with high stacking and hole mobility. Polym. Chem. 2015, 6, 7377–7388. [Google Scholar] [CrossRef]
- Villafiorita-Monteleone, F.; Cappelli, A.; Paolino, M.; Colombo, M.; Cariati, E.; Mura, A.; Bongiovanni, G.; Botta, C. Aggregation-Induced Förster Resonance Energy Transfer in Polybenzofulvene/Dye Nanoparticles. J. Phys. Chem. C 2015, 119, 18986–18991. [Google Scholar] [CrossRef]
- Cappelli, A.; Villafiorita-Monteleone, F.; Grisci, G.; Paolino, M.; Razzano, V.; Fabio, G.; Giuliani, G.; Donati, A.; Mendichi, R.; Boccia, A.C.; et al. Highly emissive supramolecular assemblies based on π-stacked polybenzofulvene hosts and a benzothiadiazole guest. J. Mater. Chem. C 2014, 2, 7897–7905. [Google Scholar] [CrossRef]
- Cappelli, A.; Grisci, G.; Paolino, M.; Razzano, V.; Giuliani, G.; Donati, A.; Bonechi, C.; Mendichi, R.; Boccia, A.C.; Licciardi, M.; et al. Polybenzofulvene derivatives bearing dynamic binding sites as potential anticancer drug delivery systems. J. Mater. Chem. B 2015, 3, 361–374. [Google Scholar] [CrossRef]
- Mrόz, W.; Villafiorita-Monteleone, F.; Pasini, M.; Grisci, G.; Paolino, M.; Razzano, V.; Cappelli, A.; Botta, C. π-stacked polybenzofulvene derivatives as hosts for yellow and red emitting OLEDs. Mater. Lett. 2015, 142, 197–200. [Google Scholar] [CrossRef]
- Paolino, M.; Grisci, G.; Castriconi, F.; Reale, A.; Giuliani, G.; Donati, A.; Bonechi, C.; Giorgi, G.; Mendichi, R.; Piovani, D.; et al. Densely PEGylated polybenzofulvene brushes for potential applications in drug encapsulation. Pharmaceutics 2018, 10, 234. [Google Scholar] [CrossRef] [Green Version]
- Cappelli, A.; Paolino, M.; Grisci, G.; Razzano, V.; Giuliani, G.; Donati, A.; Bonechi, C.; Mendichi, R.; Battiato, S.; Samperi, F.; et al. Hyaluronan-coated polybenzofulvene brushes as biomimetic materials. Polym. Chem. 2016, 7, 6529–6544. [Google Scholar] [CrossRef]
- Licciardi, M.; Scialabba, C.; Giammona, G.; Paolino, M.; Razzano, V.; Grisci, G.; Giuliani, G.; Makovec, F.; Cappelli, A. Design and development of hyaluronan-functionalized polybenzofulvene nanoparticles as CD44 receptor mediated drug delivery system. J. Nanoparticle Res. 2017, 19. [Google Scholar] [CrossRef]
- Licciardi, M.; Scialabba, C.; Sardo, C.; Cavallaro, G.; Giammona, G. Amphiphilic inulin graft co-polymers as self-assembling micelles for doxorubicin delivery. J. Mater. Chem. B 2014, 2, 4262–4271. [Google Scholar] [CrossRef]
- Bongiovì, F.; Di Prima, G.; Palumbo, F.S.; Licciardi, M.; Pitarresi, G.; Giammona, G. Hyaluronic Acid-Based Micelles as Ocular Platform to Modulate the Loading, Release, and Corneal Permeation of Corticosteroids. Macromol. Biosci. 2017, 17, 1700261. [Google Scholar] [CrossRef] [PubMed]
- Di Prima, G.; Bongiovì, F.; Palumbo, F.S.; Pitarresi, G.; Licciardi, M.; Giammona, G. Mucoadhesive PEGylated inulin-based self-assembling nanoparticles: In vitro and ex vivo transcorneal permeation enhancement of corticosteroids. J. Drug Deliv. Sci. Technol. 2019, 49, 195–208. [Google Scholar] [CrossRef]
- de Mohac, L.M.; de Fátima Pina, M.; Raimi-Abraham, B.T. Solid microcrystalline dispersion films as a new strategy to improve the dissolution rate of poorly water soluble drugs: A case study using olanzapine. Int. J. Pharm. 2016, 508, 42–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Telford, J.E.; Bones, J.; McManus, C.; Saldova, R.; Manning, G.; Doherty, M.; Leweke, F.M.; Rothermundt, M.; Guest, P.C.; Rahmoune, H.; et al. Antipsychotic Treatment of Acute Paranoid Schizophrenia Patients with Olanzapine Results in Altered Glycosylation of Serum Glycoproteins. J. Proteome Res. 2012, 11, 3743–3752. [Google Scholar] [CrossRef]
- Goswami, L.N.; Houston, Z.H.; Sarma, S.J.; Jalisatgi, S.S.; Hawthorne, M.F. Efficient synthesis of diverse heterobifunctionalized clickable oligo(ethylene glycol) linkers: Potential applications in bioconjugation and targeted drug delivery. Org. Biomol. Chem. 2013, 11, 1116–1126. [Google Scholar] [CrossRef] [Green Version]
- Leone, G.; Consumi, M.; Franzi, C.; Tamasi, G.; Lamponi, S.; Donati, A.; Magnani, A.; Rossi, C.; Bonechi, C. Development of liposomal formulations to potentiate natural lovastatin inhibitory activity towards 3-hydroxy-3-methyl-glutaryl coenzyme A (HMG-CoA) reductase. J. Drug Deliv. Sci. Technol. 2018, 43, 107–112. [Google Scholar] [CrossRef]
- Liang, L.; Astruc, D. The copper(I)-catalyzed alkyne-azide cycloaddition (CuAAC) “click” reaction and its applications. An overview. Coord. Chem. Rev. 2011, 255, 2933–2945. [Google Scholar] [CrossRef]
- Paolino, M.; Reale, A.; Razzano, V.; Giuliani, G.; Donati, A.; Bonechi, C.; Caselli, G.; Visintin, M.; Makovec, F.; Scialabba, C.; et al. Nanoreactors for the multi-functionalization of poly-histidine fragments. New J. Chem. 2019, 6834–6837. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, M.; Zhang, Y.; Zhou, H. Fluorescence Studies on Hydrophobic Associations of Fluorocarbon-Modified Poly(acrylic acid) Solutions. Macromolecules 1999, 32, 4861–4866. [Google Scholar] [CrossRef]
- Cavallari, C.; Fini, A.; Ceschel, G. Design of Olanzapine/Lutrol Solid Dispersions of Improved Stability and Performances. Pharmaceutics 2013, 5, 570–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abouelmagd, S.A.; Sun, B.; Chang, A.C.; Ku, Y.J.; Yeo, Y. Release Kinetics Study of Poorly Water-Soluble Drugs from Nanoparticles: Are We Doing It Right? Mol. Pharm. 2015, 12, 997–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malich, G.; Markovic, B.; Winder, C. The sensitivity and specificity of the MTS tetrazolium assay for detecting the in vitro cytotoxicity of 20 chemicals using human cell lines. Toxicology 1997, 124, 179–192. [Google Scholar] [CrossRef]
- Sardo, C.; Farra, R.; Licciardi, M.; Dapas, B.; Scialabba, C.; Giammona, G.; Grassi, M.; Grassi, G.; Cavallaro, G. Development of a simple, biocompatible and cost-effective Inulin-Diethylenetriamine based siRNA delivery system. Eur. J. Pharm. Sci. 2015, 75, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Guo, J.; Huang, H.; Xia, B.; Liu, H.; Li, J.; Lin, S.; Li, T.; Liu, J.; Li, H. Human Normal Bronchial Epithelial Cells: A Novel In Vitro Cell Model for Toxicity Evaluation. PLoS ONE 2015, 10, 1–14. [Google Scholar] [CrossRef]
- Xia, B.; Yang, L.; Huang, H.; Pang, L.; Hu, G.; Liu, Q.; Yuan, J.; Liu, J.; Xia, Y.; Zhuang, Z. Chromium(VI) causes down regulation of biotinidase in human bronchial epithelial cells by modifications of histone acetylation. Toxicol. Lett. 2011, 205, 140–145. [Google Scholar] [CrossRef]
- Forbes, B.; Shah, A.; Martin, G.P.; Lansley, A.B. The human bronchial epithelial cell line 16HBE14o− as a model system of the airways for studying drug transport. Int. J. Pharm. 2003, 257, 161–167. [Google Scholar] [CrossRef]














| Samples | Size (nm) | PDI | Z-potential (mV) |
|---|---|---|---|
| HA-FA-HEG-OA | 291 | 0.424 | −47 |
| HA-FA-HEG-SA | 364 | 0.442 | −35 |
| Samples | Size (nm) | PDI | Z-Potential (mV) | Drug Loading (%) |
|---|---|---|---|---|
| HA-FA-HEG-OA-OZ | 234 | 0.352 | −31.1 | 14.0 |
| HA-FA-HEG-SA-OZ | 178 | 0.260 | −24.7 | 14.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paolino, M.; Licciardi, M.; Savoca, C.; Giammona, G.; Modica De Mohac, L.; Reale, A.; Giuliani, G.; Komber, H.; Donati, A.; Leone, G.; et al. Hyaluronan Graft Copolymers Bearing Fatty-Acid Residues as Self-Assembling Nanoparticles for Olanzapine Delivery. Pharmaceutics 2019, 11, 675. https://doi.org/10.3390/pharmaceutics11120675
Paolino M, Licciardi M, Savoca C, Giammona G, Modica De Mohac L, Reale A, Giuliani G, Komber H, Donati A, Leone G, et al. Hyaluronan Graft Copolymers Bearing Fatty-Acid Residues as Self-Assembling Nanoparticles for Olanzapine Delivery. Pharmaceutics. 2019; 11(12):675. https://doi.org/10.3390/pharmaceutics11120675
Chicago/Turabian StylePaolino, Marco, Mariano Licciardi, Cristina Savoca, Gaetano Giammona, Laura Modica De Mohac, Annalisa Reale, Germano Giuliani, Hartmut Komber, Alessandro Donati, Gemma Leone, and et al. 2019. "Hyaluronan Graft Copolymers Bearing Fatty-Acid Residues as Self-Assembling Nanoparticles for Olanzapine Delivery" Pharmaceutics 11, no. 12: 675. https://doi.org/10.3390/pharmaceutics11120675