Using a Material Library to Understand the Impacts of Raw Material Properties on Ribbon Quality in Roll Compaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Powder Characterization
2.3. Roll Compaction and Ribbon Production
2.4. Ribbon Characterization
2.4.1. Ribbon Solid Fraction
2.4.2. Ribbon Tensile Strength
2.4.3. Ribbon Morphology
2.5. Empirical Model Fitting for Roll Compaction
2.5.1. The Ryshkewitch–Duckworth Model
2.5.2. The Power Model
2.6. Multivariate Analysis
3. Results and Discussion
3.1. The Powder Properties
3.2. The Ribbon Characteristics
3.3. Description of Roll Compaction Behavior
3.3.1. Relationship between Ribbon Tensile Strength and Porosity
3.3.2. Relationship between Ribbon Tensile Strength and Hydraulic Pressure
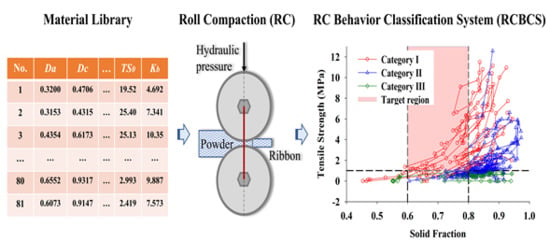
3.4. Roll Compaction Behavior Classification System
3.5. Latent Variable Modeling in Prediction of Ribbon Properties
3.6. Development of Multi-Objective Design Space
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boersen, N.; Belair, D.; Peck, G.E.; Pinal, R. A dimensionless variable for the scale up and transfer of a roller compaction formulation. Drug Dev. Ind. Pharm. 2016, 42, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Falzone, A.M. Effects of changes in roller compactor parameters on granulations produced by compaction. Drug Dev. Ind. Pharm. 2008, 18, 469–489. [Google Scholar] [CrossRef]
- Inghelbrecht, S.; Remon, J.P.; de Aguiar, P.F.; Walczak, B.; Massart, D.; Van De Velde, F.; Hans, V.P.B. Instrumentation of a roll compactor and the evaluation of the parameter setting by neural networks. Int. J. Pharm. 1997, 148, 103–115. [Google Scholar] [CrossRef]
- Kleinebudde, P. Roll compaction/dry granulation: Pharmaceutical applications. Eur. J. Pharm. Biopharm. 2004, 58, 317–326. [Google Scholar] [CrossRef]
- Sakwanichol, J.; Puttipipatkhachorn, S.; Ingenerf, G.; Kleinebudde, P. Roll compaction/dry granulation: Comparison between roll mill and oscillating granulator in dry granulation. Pharm. Dev. Technol. 2012, 17, 30–39. [Google Scholar] [CrossRef]
- Khan, A. Optimization of the process variables of roller compaction, on the basis of granules characteristics (flow, mechanical strength, and disintegration behavior): An application of SeDeM-ODT expert system. Drug Dev. Ind. Pharm. 2019, 45, 1537–1546. [Google Scholar] [CrossRef]
- Teng, Y.; Qiu, Z.; Wen, H. Systematical approach of formulation and process development using roller compaction. Eur. J. Pharm. Biopharm. 2009, 73, 219–229. [Google Scholar] [CrossRef]
- Leane, M.; Pitt, K.; Reynolds, G.K.; Dawson, N.; Ziegler, I.; Szepes, A.; Crean, A.M.; Dall Agnol, R. The Manufacturing Classification System Mc, S.W.G. Manufacturing classification system in the real world: Factors influencing manufacturing process choices for filed commercial oral solid dosage formulations, case studies from industry and considerations for continuous processing. Pharm. Dev. Technol. 2018, 23, 964–977. [Google Scholar] [CrossRef]
- Sabine Inghelbrecht, J.P.R. Reducing dust and improving granule and tablet quality in the roller compaction process. Int. J. Pharm. 1998, 171, 195–206. [Google Scholar] [CrossRef]
- Patel, S.; Kaushal, A.M.; Bansal, A.K. Compaction behavior of roller compacted ibuprofen. Eur. J. Pharm. Biopharm. 2008, 69, 743–749. [Google Scholar] [CrossRef]
- Kuntz, T.; Schubert, M.A.; Kleinebudde, P. Increased compactibility of acetames after roll compaction. Eur. J. Pharm. Biopharm. 2011, 77, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Omar, C.S.; Dhenge, R.M.; Osborne, J.D.; Althaus, T.O.; Palzer, S.; Hounslow, M.J.; Salman, A.D. Roller compaction: Effect of morphology and amorphous content of lactose powder on product quality. Int. J. Pharm. 2015, 496, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Heng, P.W.; Chan, L.W.; Liew, C.V.; Chee, S.N.; Soh, J.L.; Ooi, S.M. Roller compaction of crude plant material: Influence of process variables, polyvinylpyrrolidone, and co-milling. Pharm. Dev. Technol. 2004, 9, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, A.D.; Reynolds, G.K.; Wilson, D.; Wren, S.A.C.; Salman, A.D. The effect of roller compaction and tableting stresses on pharmaceutical tablet performance. Powder Technol. 2019, 341, 23–37. [Google Scholar] [CrossRef]
- Yoshiro Funakoshi, T.A.; Eiichi, S. The use of a novel compactor with a concavo-convex roller pair to obtain uniform compacting pressure. Drug Dev. Ind. Pharm. 1977, 3, 19. [Google Scholar] [CrossRef]
- Jaminet, F.; Hess, H. Studies on compacting and dry granulation. Pharm. Acta Helv. 1966, 41, 39. [Google Scholar]
- Sun, C.C.; Kleinebudde, P. Mini review: Mechanisms to the loss of tabletability by dry granulation. Eur. J. Pharm. Biopharm. 2016, 106, 9–14. [Google Scholar] [CrossRef]
- Wiedey, R.; Sibanc, R.; Wilms, A.; Kleinebudde, P. How relevant is ribbon homogeneity in roll compaction/dry granulation and can it be influenced? Eur. J. Pharm. Biopharm. 2018, 133, 232–239. [Google Scholar] [CrossRef]
- Bultmann, J.M. Multiple compaction of microcrystalline cellulose in a roller compactor. Eur. J. Pharm. Biopharm. 2002, 54, 59–64. [Google Scholar] [CrossRef]
- Sabine Inghelbrecht, J.P.R. Roller compaction and tableting of microcrystalline cellulose/drug mixtures. Int. J. Pharm. 1998, 161, 215–224. [Google Scholar] [CrossRef]
- Reynolds, G.; Ingale, R.; Roberts, R.; Kothari, S.; Gururajan, B. Practical application of roller compaction process modeling. Comput. Chem. Eng. 2010, 34, 1049–1057. [Google Scholar] [CrossRef]
- Liu, Y.; Wassgren, C. Modifications to Johanson’s roll compaction model for improved relative density predictions. Powder Technol. 2016, 297, 294–302. [Google Scholar] [CrossRef] [Green Version]
- Bindhumadhavan, G.; Seville, J.P.K.; Adams, M.J.; Greenwood, R.W.; Fitzpatrick, S. Roll compaction of a pharmaceutical excipient: Experimental validation of rolling theory for granular solids. Chem. Eng. Sci. 2005, 60, 3891–3897. [Google Scholar] [CrossRef]
- Johanson, J.R. A Rolling Theory for Granular Solids. J. Appl. Mech. 1965, 32, 842. [Google Scholar] [CrossRef]
- Kornienko, P.A.; Pugin, V.S. Theory and technology of the component formation process. Powder Metall. Metal Ceram. 1966, 8, 26–28. [Google Scholar] [CrossRef]
- Peter, S.; Lammens, R.F.; Steffens, K.-J. Roller compaction/Dry granulation: Use of the thin layer model for predicting densities and forces during roller compaction. Powder Technol. 2010, 199, 165–175. [Google Scholar] [CrossRef]
- Muliadi, A.R.; Litster, J.D.; Wassgren, C.R. Modeling the powder roll compaction process: Comparison of 2-D finite element method and the rolling theory for granular solids (Johanson’s model). Powder Technol. 2012, 221, 90–100. [Google Scholar] [CrossRef]
- Nesarikar, V.V.; Patel, C.; Early, W.; Vatsaraj, N.; Sprockel, O.; Jerzweski, R. Roller compaction process development and scale up using Johanson model calibrated with instrumented roll data. Int. J. Pharm. 2012, 436, 486–507. [Google Scholar] [CrossRef]
- Dec, R.T.; Zavaliangos, A.; Cunningham, J.C. Comparison of various modeling methods for analysis of powder compaction in roller press. Powder Technol. 2003, 130, 265–271. [Google Scholar] [CrossRef]
- Egbu, R.; Davies, C.; Dawson, N.; Coupe, A. Linking API brittleness in high load formulations to the roller compaction process. Powder Technol. 2018, 328, 337–344. [Google Scholar] [CrossRef]
- Wagner, C.M.; Pein, M.; Breitkreutz, J. Roll compaction of mannitol: Compactability study of crystalline and spray-dried grades. Int. J. Pharm. 2013, 453, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Gururajan, B.; Reynolds, G.; Roberts, R.; Adams, M.J.; Wu, C.Y. A comparative study of roll compaction of free-flowing and cohesive pharmaceutical powders. Int. J. Pharm. 2012, 428, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Mansa, R.F.; Bridson, R.H.; Greenwood, R.W.; Barker, H.; Seville, J.P.K. Using intelligent software to predict the effects of formulation and processing parameters on roller compaction. Powder Technol. 2008, 181, 217–225. [Google Scholar] [CrossRef]
- Soh, J.L.; Wang, F.; Boersen, N.; Pinal, R.; Peck, G.E.; Carvajal, M.T.; Cheney, J.; Valthorsson, H.; Pazdan, J. Utility of multivariate analysis in modeling the effects of raw material properties and operating parameters on granule and ribbon properties prepared in roller compaction. Drug Dev. Ind. Pharm. 2008, 34, 1022–1035. [Google Scholar] [CrossRef] [PubMed]
- Pishnamazi, M.; Casilagan, S.; Clancy, C.; Shirazian, S.; Iqbal, J.; Egan, D.; Edlin, C.; Croker, D.M.; Walker, G.M.; Collins, M.N. Microcrystalline cellulose, lactose and lignin blends: Process mapping of dry granulation via roll compaction. Powder Technol. 2019, 341, 38–50. [Google Scholar] [CrossRef]
- Souihi, N.; Dumarey, M.; Wikstrom, H.; Tajarobi, P.; Fransson, M.; Svensson, O.; Josefson, M.; Trygg, J. A quality by design approach to investigate the effect of mannitol and dicalcium phosphate qualities on roll compaction. Int. J. Pharm. 2013, 447, 47–61. [Google Scholar] [CrossRef]
- Mangal, H.; Kirsolak, M.; Kleinebudde, P. Roll compaction/dry granulation: Suitability of different binders. Int. J. Pharm. 2016, 503, 213–219. [Google Scholar] [CrossRef]
- Al-Asady, R.B.; Osborne, J.D.; Hounslow, M.J.; Salman, A.D. Roller compactor: The effect of mechanical properties of primary particles. Int. J. Pharm. 2015, 496, 124–136. [Google Scholar] [CrossRef]
- Al Asady, R.B.; Hounslow, M.J.; Salman, A.D. Roller compaction: The effect of plastic deformation of primary particles with wide range of mechanical properties. Drug Deliv. Transl. Res. 2018, 8, 1615–1634. [Google Scholar] [CrossRef]
- Wang, T.; Alston, K.M. The Creation of an Excipient Properties Database to Support Quality by Design (QbD) Formulation Development. Am. Pharm. Rev. 2013, 16, 16–25. [Google Scholar]
- Escotet-Espinoza, M.S.; Moghtadernejad, S.; Scicolone, J.; Wang, Y.; Pereira, G.; Schäfer, E.; Vigh, T.; Klingeleers, D.; Ierapetritou, M.; Muzzio, F.J. Using a material property library to find surrogate materials for pharmaceutical process development. Powder Technol. 2018, 339, 659–676. [Google Scholar] [CrossRef]
- Van Snick, B.; Dhondt, J.; Pandelaere, K.; Bertels, J.; Mertens, R.; Klingeleers, D.; Di Pretoro, G.; Remon, J.P.; Vervaet, C.; De Beer, T.; et al. A multivariate raw material property database to facilitate drug product development and enable in-silico design of pharmaceutical dry powder processes. Int. J. Pharm. 2018, 549, 415–435. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, A.; Khoo, J.; Sharma, S.; Facco, P.; Barolo, M.; Zomer, S. Data analytics on raw material properties to accelerate pharmaceutical drug development. Int. J. Pharm. 2019, 563, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Oishi, T.; Shirotori, K.; Marumo, Y.; Kosugi, A.; Kumada, S.; Hirai, D.; Takayama, K.; Onuki, Y. Modeling of quantitative relationships between physicochemical properties of active pharmaceutical ingredients and tensile strength of tablets using a boosted tree. Drug Dev. Ind. Pharm. 2018, 44, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; O’Connor, T.; Li, T.; Ashraf, M.; Cruz, C.N. Development and Applications of a Material Library for Pharmaceutical Continuous Manufacturing of Solid Dosage Forms. Int. J. Pharm. 2019, 569. [Google Scholar] [CrossRef]
- Shi, Z.; Hilden, J.L. Small-Scale Modeling of Pharmaceutical Powder Compression from Tap Density Testers, to Roller Compactors, and to the Tablet Press Using Big Data. J. Pharm. Innov. 2016, 12, 41–48. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, M.; Wu, F.; Shen, L.; Lin, X.; Feng, Y. Direct compaction properties of Zingiberis Rhizoma extracted powders coated with various shell materials: Improvements and mechanism analysis. Int. J. Pharm. 2019, 564, 10–21. [Google Scholar] [CrossRef]
- Li, Z.; Wu, F.; Zhao, L.; Lin, X.; Shen, L.; Feng, Y. Evaluation of fundamental and functional properties of natural plant product powders for direct compaction based on multivariate statistical analysis. Adv. Powder Technol. 2018, 29, 2881–2894. [Google Scholar] [CrossRef]
- Dai, S.; Xu, B.; Shi, G.; Liu, J.; Zhang, Z.; Shi, X.; Qiao, Y. SeDeM expert system for directly compressed tablet formulation: A review and new perspectives. Powder Technol. 2019, 342, 517–527. [Google Scholar] [CrossRef]
- Dai, S.; Xu, B.; Zhang, Z.; Yu, J.; Wang, F.; Shi, X.; Qiao, Y. A compression behavior classification system of pharmaceutical powders for accelerating direct compression tablet formulation design. Int. J. Pharm. 2019. [Google Scholar] [CrossRef]
- McAuliffe, M.A.P.; O’Mahony, G.E.; Blackshields, C.A.; Collins, J.A.; Egan, D.P.; Kiernan, L.; O’Neill, E.; Lenihan, S.; Walker, G.M.; Crean, A.M. The Use of PAT and Off-line Methods for Monitoring of Roller Compacted Ribbon and Granule Properties with a View to Continuous Processing. Org. Process Res. Dev. 2014, 19, 158–166. [Google Scholar] [CrossRef]
- Sajjia, M.; Shirazian, S.; Egan, D.; Iqbal, J.; Albadarin, A.B.; Southern, M.; Walker, G. Mechanistic modelling of industrial-scale roller compactor ‘Freund TF-MINI model’. Comput. Chem. Eng. 2017, 104, 141–150. [Google Scholar] [CrossRef]
- Sabine Inghelbrecht, J.P.R. The roller compaction of different types of lactose. Int. J. Pharm. 1998, 166, 135–144. [Google Scholar] [CrossRef]
- Nesarikar, V.V.; Vatsaraj, N.; Patel, C.; Early, W.; Pandey, P.; Sprockel, O.; Gao, Z.; Jerzewski, R.; Miller, R.; Levin, M. Instrumented roll technology for the design space development of roller compaction process. Int. J. Pharm. 2012, 426, 116–131. [Google Scholar] [CrossRef] [PubMed]
- Khorasani, M.; Amigo, J.M.; Sun, C.C.; Bertelsen, P.; Rantanen, J. Near-infrared chemical imaging (NIR-CI) as a process monitoring solution for a production line of roll compaction and tableting. Eur. J. Pharm. Biopharm. 2015, 93, 293–302. [Google Scholar] [CrossRef]
- Yu, M.; Omar, C.; Schmidt, A.; Litster, J.D.; Salman, A.D. Improving feeding powder distribution to the compaction zone in the roller compaction. Eur. J. Pharm. Biopharm. 2018, 128, 57–68. [Google Scholar] [CrossRef]
- Zinchuk, A.V.; Mullarney, M.P.; Hancock, B.C. Simulation of roller compaction using a laboratory scale compaction simulator. Int. J. Pharm. 2004, 269, 403–415. [Google Scholar] [CrossRef]
- Mirtič, A.; Reynolds, G.K. Determination of breakage rate and breakage mode of roller compacted pharmaceutical materials. Powder Technol. 2016, 298, 99–105. [Google Scholar] [CrossRef]
- Boersen, N.; Carvajal, M.T.; Morris, K.R.; Peck, G.E.; Pinal, R. The influence of API concentration on the roller compaction process: Modeling and prediction of the post compacted ribbon, granule and tablet properties using multivariate data analysis. Drug Dev. Ind. Pharm. 2015, 41, 1470–1478. [Google Scholar] [CrossRef]
- Farber, L.; Hapgood, K.P.; Michaels, J.N.; Fu, X.Y.; Meyer, R.; Johnson, M.A.; Li, F. Unified compaction curve model for tensile strength of tablets made by roller compaction and direct compression. Int. J. Pharm. 2008, 346, 17–24. [Google Scholar] [CrossRef]
- Ryshkewitch, E. Compression Strength of Porous Sintered Alumina and Zirconia. J. Am. Ceram. Soc. 1953, 36, 65–68. [Google Scholar] [CrossRef]
- Wu, C.Y.; Best, S.M.; Bentham, A.C.; Hancock, B.C.; Bonfield, W. A simple predictive model for the tensile strength of binary tablets. Eur. J. Pharm. Biopharm. 2005, 25, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Bansal, A.K. Prediction of mechanical properties of compacted binary mixtures containing high-dose poorly compressible drug. Int. J. Pharm. 2011, 403, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Steendam, R.; Lerk, C.F. Poly(DL-lactic acid) as a direct compression excipient in controlled release tables. Part I. Compaction behaviour and release charecteristics of poly (DL-lactic acid) matrix tablets. Int. J. Pharm. 1998, 175, 33–46. [Google Scholar] [CrossRef]
- Barralet, J.E.; Gaunt, T.; Wright, A.J.; Gibson, I.R.; Knowles, J.C. Effect of porosity reduction by compaction on compressive strength and microstructure of calcium phosphate cement. J. Biomed. Mater. Res. 2001, 63, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Shin, H.S.; Lee, S.K.; Lee, B.K. Aggregate and necking force in Mn–Zn ferrite. Mater. Lett. 2003, 57, 1467–1470. [Google Scholar] [CrossRef]
- Gbureck, U. Mechanical activation and cement formation of β-tricalcium phosphate. Biomaterials 2003, 24, 4123–4131. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Best, S.M.; Bentham, A.C.; Hancock, B.C.; Bonfield, W. Predicting the Tensile Strength of Compacted Multi-Component Mixtures of Pharmaceutical Powders. Pharm. Res. 2006, 23, 1898–1905. [Google Scholar] [CrossRef]
- Gupta, A.; Peck, G.E.; Miller, R.W.; Morris, K.R. Influence of ambient moisture on the compaction behavior of microcrystalline cellulose powder undergoing uni-axial compression and roller-compaction: A comparative study using near-infrared spectroscopy. J. Pharm. Sci. 2005, 94, 2301–2313. [Google Scholar] [CrossRef]
- Osborne, J.D.; Althaus, T.; Forny, L.; Niederreiter, G.; Palzer, S.; Hounslow, M.J.; Salman, A.D. Investigating the influence of moisture content and pressure on the bonding mechanisms during roller compaction of an amorphous material. Chem. Eng. Sci. 2013, 86, 61–69. [Google Scholar] [CrossRef]
- Wold, S. Principal Component Analysis. Chemom. Intell. Lab. Syst. 1987, 2, 37–52. [Google Scholar] [CrossRef]
- Wold, S.; Trygg, J.; Berglund, A.; Antti, H. Anders Berglund, Henrik Antti. Some recent developments in PLS modeling. Chemom. Intell. Lab. Syst. 2001, 58, 131–150. [Google Scholar] [CrossRef]
- Nofrerias, I.; Nardi, A.; Suñé-Pou, M.; Boeckmans, J.; Suñé-Negre, J.M.; García-Montoya, E. Optimization of the Cohesion Index in the SeDeM Diagram Expert System and application of SeDeM Diagram: An improved methodology to determine the Cohesion Index. PLoS ONE 2018, 13, e0203846. [Google Scholar] [CrossRef] [PubMed]
- Mahmah, O.; Adams, M.J.; Omar, C.S.; Gururajan, B.; Salman, A.D. Roller compaction: Ribbon splitting and sticking. Int. J. Pharm. 2019, 559, 156–172. [Google Scholar] [CrossRef] [Green Version]
- Allesø, M.; Holm, R.; Holm, P. Roller compaction scale-up using roll width as scale factor and laser-based determined ribbon porosity as critical material attribute. Eur. J. Pharm. Sci. 2015, 87, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Miguelez-Moran, A.M.; Wu, C.Y.; Dong, H.; Seville, J.P. Characterisation of density distributions in roller-compacted ribbons using micro-indentation and X-ray micro-computed tomography. Eur. J. Pharm. Biopharm. 2009, 72, 173–182. [Google Scholar] [CrossRef]
- Wiedey, R.; Kleinebudde, P. Infrared thermography—A new approach for in-line density measurement of ribbons produced from roll compaction. Powder Technol. 2018, 337, 17–24. [Google Scholar] [CrossRef]
- Saleh, T.A. Surface Enhanced Raman Scattering Spectroscopy for Pharmaceutical Determination. Int. J. Nanomater. Nanotechnol. Nanomed. 2016. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, A.; Saleh, T.A.; Abulkibash, A.M.; Ibrahim, K.E. Chemometric Optimization of Sequential Injection Spectrophotometric Method for Chlorpheniramine Determination in Pharmaceutical Formulations. J. Flow Inject. Anal. 2010, 27, 26–31. [Google Scholar]
- Idris, A.M.; Ibrahim, A.E.; Abulkibash, A.M.; Saleh, T.A.; Ibrahim, K.E. Rapid inexpensive assay method for verapamil by spectrophotometric sequential injection analysis. Drug Test. Anal. 2011, 3, 380–386. [Google Scholar] [CrossRef]
- Katz, J.M.; Buckner, I.S. Full Out-of-Die Compressibility and Compactibility Profiles from Two Tablets. J. Pharm. Sci. 2017, 106, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.C. Mechanism of moisture induced variations in true density and compaction properties of microcrystalline cellulose. Int. J. Pharm. 2008, 346, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Hancock, B.C.; Colvin, J.T.; Mullarney, M.P.; Zinchuk, A.V. The Relative Densities of Pharmaceutical Powders, Blends, Dry Granulations, and Immediate-Release Tablets. Pharm. Technol. 2003, 4, 64–80. [Google Scholar]
- Leane, M.; Pitt, K.; Reynolds, G. Manufacturing Classification System Working, G. A proposal for a drug product Manufacturing Classification System (MCS) for oral solid dosage forms. Pharm. Dev. Technol. 2015, 20, 12–21. [Google Scholar] [CrossRef]
- He, X.; Secreast, P.J.; Amidon, G.E. Mechanistic study of the effect of roller compaction and lubricant on tablet mechanical strength. J. Pharm. Sci. 2007, 96, 1342–1355. [Google Scholar] [CrossRef]
- Herting, M.G.; Kleinebudde, P. Roll compaction/dry granulation: Effect of raw material particle size on granule and tablet properties. Int. J. Pharm. 2007, 338, 110–118. [Google Scholar] [CrossRef]
- Queiroz, A.L.P.; Faisal, W.; Devine, K.; Garvie-Cook, H.; Vucen, S.; Crean, A.M. The application of percolation threshold theory to predict compaction behaviour of pharmaceutical powder blends. Powder Technol. 2019, 354, 188–198. [Google Scholar] [CrossRef]
- Grote, S.; Kleinebudde, P. Impact of functionalized particle structure on roll compaction/dry granulation and tableting of calcium carbonate. Int. J. Pharm. 2018, 544, 235–241. [Google Scholar] [CrossRef]
- Bro, R. Multivariate calibration: What is in chemometrics for the analytical chemist? Anal. Chim. Acta 2003, 500, 185–194. [Google Scholar] [CrossRef]
- Dumarey, M.; Wikstrom, H.; Fransson, M.; Sparen, A.; Tajarobi, P.; Josefson, M.; Trygg, J. Combining experimental design and orthogonal projections to latent structures to study the influence of microcrystalline cellulose properties on roll compaction. Int. J. Pharm. 2011, 416, 110–119. [Google Scholar] [CrossRef]
- Boersen, N. The Development of Roller Compacted Formulations Using Multivaraite and Dimensional Analysis. Ph.D. Thesis, Purdue University, West Lafayette, IA, USA, 2010. [Google Scholar]
- Megarry, A.J.; Swainson, S.M.E.; Roberts, R.J.; Reynolds, G.K. A big data approach to pharmaceutical flow properties. Int. J. Pharm. 2019, 555, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Tajarobi, P.; Boissier, C.; Sun, C.C. Tableting performance of various mannitol and lactose grades assessed by compaction simulation and chemometrical analysis. Int. J. Pharm. 2019, 566, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Reimer, H.L.; Kleinebudde, P. Hybrid modeling of roll compaction processes with the Styl’One Evolution. Powder Technol. 2019, 341, 66–74. [Google Scholar] [CrossRef]
- Escotet-Espinoza, M.S.; Vadodaria, S.; Singh, R.; Muzzio, F.J.; Ierapetritou, M.G. Modeling the effects of material properties on tablet compaction: A building block for controlling both batch and continuous pharmaceutical manufacturing processes. Int. J. Pharm. 2018, 543, 274–287. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Hung, W.L.; Miguelez-Moran, A.M.; Gururajan, B.; Seville, J.P. Roller compaction of moist pharmaceutical powders. Int. J. Pharm. 2010, 391, 90–97. [Google Scholar] [CrossRef]
- Peltonen, L. Design Space and QbD Approach for Production of Drug Nanocrystals by Wet Media Milling Techniques. Pharmaceutics 2018, 10, 104. [Google Scholar] [CrossRef] [Green Version]










| Splitting Mode | Splitting Occurrences Under Different Pressures | |||||
|---|---|---|---|---|---|---|
| 30 bar | 50 bar | 70 bar | 90 bar | 110 bar | ||
| T | SUM | 39 | 40 | 34 | 31 | 26 |
| L | L2 | 4 | 15 | 20 | 23 | 23 |
| L3 | 0 | 0 | 1 | 1 | 0 | |
| LJ | 3 | 8 | 18 | 19 | 22 | |
| LN | 3 | 3 | 4 | 5 | 4 | |
| SUM | 10 | 26 | 43 | 48 | 49 | |
| Main Category | Main Criteria | Subcategory | Second Criteria | No. of Excipients | No. of NPPs |
|---|---|---|---|---|---|
| Ⅰ | 0.6 ≤ SF ≤ 0.8 and TS ≥ 1 MPa | ⅠA | Fulfilling the main criteria at 30–70 bar | 21 | 5 |
| ⅠB | Fulfilling the main criteria at 90–110 bar | 3 | 0 | ||
| Ⅱ | SF > 0.8 and TS ≥ 1 MPa | ⅡA | Fulfilling the main criteria at 30–70 bar | 14 | 12 |
| ⅡB | Fulfilling the main criteria at 90–110 bar | 7 | 8 | ||
| Ⅲ | TS < 1 MPa | / | / | 8 | 3 |
| Variable Type | ||
|---|---|---|
| Input | Material property | D10, D50,D90, span, %Pf, Iθ, Da, Dc, Dt, SFp, εp, IH, IC, Ie, t″, AOR, %HR, %H, Icd, Co, Sp, Sp-co |
| Compaction descriptor | kb, TS0, d, g | |
| Hydraulic pressure | P | |
| Output | Ribbon property | TS, SF |
| Model | LVs | R2Xcum | R2Ycum | Q2Ycum |
|---|---|---|---|---|
| 1 | 4 | 71.9% | 54.7% | 51.1% |
| 2 | 4 | 95.5% | 67.5% | 64.9% |
| 3 | 5 | 70.8% | 75.6% | 71.8% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Xu, B.; Zhang, K.; Shi, C.; Zhang, Z.; Fu, J.; Qiao, Y. Using a Material Library to Understand the Impacts of Raw Material Properties on Ribbon Quality in Roll Compaction. Pharmaceutics 2019, 11, 662. https://doi.org/10.3390/pharmaceutics11120662
Yu J, Xu B, Zhang K, Shi C, Zhang Z, Fu J, Qiao Y. Using a Material Library to Understand the Impacts of Raw Material Properties on Ribbon Quality in Roll Compaction. Pharmaceutics. 2019; 11(12):662. https://doi.org/10.3390/pharmaceutics11120662
Chicago/Turabian StyleYu, Jiaqi, Bing Xu, Kunfeng Zhang, Chenfeng Shi, Zhiqiang Zhang, Jing Fu, and Yanjiang Qiao. 2019. "Using a Material Library to Understand the Impacts of Raw Material Properties on Ribbon Quality in Roll Compaction" Pharmaceutics 11, no. 12: 662. https://doi.org/10.3390/pharmaceutics11120662