A Novel Hybrid Additive Manufacturing Process for Drug Delivery Systems with Locally Incorporated Drug Depots
Abstract
:1. Introduction
2. Materials and Methods
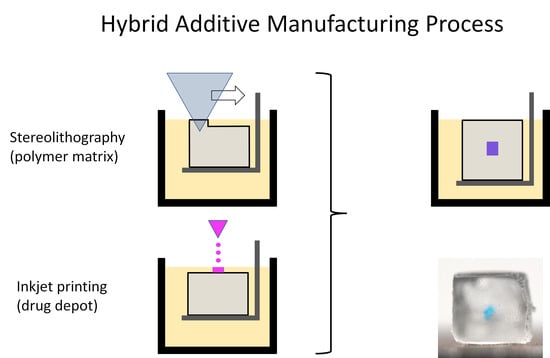
2.1. Hybrid Additive Manufacturing Process
2.1.1. Technical Concept
2.1.2. Printing Strategy
2.2. Preliminary Experimental Study
2.2.1. Photopolymer and Parameters for SLA Printing
2.2.2. Test Liquids for IJP
2.2.3. Single and Multiple Depots
2.2.4. Long-Term Diffusion Study
2.2.5. DDS with Incorporated Model Drug Depot
3. Results
3.1. Hybrid Additive Manufacturing System
3.2. Experimental Studies
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Palo, M.; Holländer, J.; Suominen, J.; Yliruusi, J.; Sandler, N. 3D printed drug delivery devices: Perspectives and technical challenges. Expert Rev. Med. Devices 2017, 14, 685–696. [Google Scholar] [CrossRef]
- Konta, A.A.; García-Piña, M.; Serrano, D.R. Personalised 3D Printed Medicines: Which Techniques and Polymers Are More Successful? Bioengineering 2017, 4, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Sing, S.L.; An, J.; Yeong, W.Y.; Wiria, F.E. Laser and electron-beam powder-bed additive manufacturing of metallic implants: A review on processes, materials and designs. J. Orthop. Res. 2016, 34, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Jamróz, W.; Szafraniec, J.; Kurek, M.; Jachowicz, R. 3D Printing in Pharmaceutical and Medical Applications-Recent Achievements and Challenges. Pharm. Res. 2018, 35, 176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucking, T.M.; Hill, E.R.; Robertson, J.L.; Maneas, E.; Plumb, A.A.; Nikitichev, D.I. From medical imaging data to 3D printed anatomical models. PLoS ONE 2017, 12, e0178540. [Google Scholar] [CrossRef] [Green Version]
- Fredieu, R.; Kerbo, J.; Herron, M.; Klatte, R.; Cooke, M. Anatomical Models: A Digital Revolution. Med Sci. Educ. 2015, 25, 183–194. [Google Scholar] [CrossRef] [Green Version]
- Mota, C.; Puppi, D.; Chiellini, F.; Chiellini, E. Additive manufacturing techniques for the production of tissue engineering constructs. J. Tissue Eng. Regen. Med. 2012, 9, 174–190. [Google Scholar] [CrossRef]
- Chartrain, N.A.; Williams, C.B.; Whittington, A.R. A review on fabricating tissue scaffolds using vat photopolymerization. Acta Biomater. 2018, 74, 90–111. [Google Scholar] [CrossRef]
- Heller, M.; Bauer, H.-K.; Goetze, E.; Gielisch, M.; Ozbolat, I.T.; Moncal, K.K.; Rizk, E.; Seitz, H.; Gelinsky, M.; Schröder, H.C.; et al. Materials and scaffolds in medical 3D printing and bioprinting in the context of bone regeneration. Int. J. Comput. Dent. 2016, 19, 301–321. [Google Scholar]
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioact. Mater. 2018, 3, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Qiu, K.; Haghiashtiani, G.; McAlpine, M.C. 3D Printed Organ Models for Surgical Applications. Annu. Rev. Anal. Chem. (Palo Alto Calif.) 2018, 11, 287–306. [Google Scholar] [CrossRef] [PubMed]
- FDA Application Number: 207958Orig1s000 Approval Letter. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/207958Orig1s000Approv.pdf (accessed on 25 October 2019).
- Wang, J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Stereolithographic (SLA) 3D printing of oral modified-release dosage forms. Int. J. Pharm. 2016, 503, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Vehse, M.; Petersen, S.; Sternberg, K.; Schmitz, K.-P.; Seitz, H. Drug Delivery From Poly(ethylene glycol) Diacrylate Scaffolds Produced by DLC Based Micro-Stereolithography. Macromol. Symp. 2014, 346, 43–47. [Google Scholar] [CrossRef]
- Pereira, R.F.; Bártolo, P.J. 3D Photo-Fabrication for Tissue Engineering and Drug Delivery. Engineering 2015, 1, 90–112. [Google Scholar] [CrossRef] [Green Version]
- Rekowska, N.; Arbeiter, D.; Konasch, J.; Riess, A.; Mau, R.; Eickner, T.; Seitz, H.; Grabow, N.; Teske, M. Thermomechanical properties of PEGDA and its co-polymers. Curr. Dir. Biomed. Eng. 2018, 4, 669–672. [Google Scholar] [CrossRef]
- Choi, J.-W.; Kim, H.-C.; Wicker, R. Multi-material stereolithography. J. Mater. Process. Technol. 2011, 211, 318–328. [Google Scholar] [CrossRef]
- Arcaute, K.; Mann, B.; Wicker, R. Stereolithography of spatially controlled multi-material bioactive poly(ethylene glycol) scaffolds. Acta Biomater. 2010, 6, 1047–1054. [Google Scholar] [CrossRef]
- Kim, Y.T.; Castro, K.; Bhattacharjee, N.; Folch, A. Digital Manufacturing of Selective Porous Barriers in Microchannels Using Multi-Material Stereolithography. Micromachines 2018, 9, 125. [Google Scholar] [CrossRef] [Green Version]
- Robles-Martinez, P.; Xu, X.; Trenfield, S.J.; Awad, A.; Goyanes, A.; Telford, R.; Basit, A.W.; Gaisford, S. 3D Printing of a Multi-Layered Polypill Containing Six Drugs Using a Novel Stereolithographic Method. Pharmaceutics 2019, 11, 274. [Google Scholar] [CrossRef] [Green Version]
- Scoutaris, N.; Alexander, M.R.; Gellert, P.R.; Roberts, C.J. Inkjet printing as a novel medicine formulation technique. J. Control. Release 2011, 156, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Scoutaris, N.; Ross, S.; Douroumis, D. Current Trends on Medical and Pharmaceutical Applications of Inkjet Printing Technology. Pharm. Res. 2016, 33, 1799–1816. [Google Scholar] [CrossRef] [PubMed]
- Daly, R.; Harrington, T.S.; Martin, G.D.; Hutchings, I.M. Inkjet printing for pharmaceutics—A review of research and manufacturing. Int. J. Pharm. 2015, 494, 554–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konasch, J.; Riess, A.; Teske, M.; Rekowska, N.; Mau, R.; Eickner, T.; Grabow, N.; Seitz, H. Novel 3D printing concept for the fabrication of time-controlled drug delivery systems. Curr. Dir. Biomed. Eng. 2018, 4, 141–144. [Google Scholar] [CrossRef]
- Vehse, M.; Seitz, H. A new Micro-Stereolithography-System based on Diode Laser Curing (DLC). Int. J. Precis. Eng. Manuf. 2014, 15, 2161–2166. [Google Scholar] [CrossRef]
- Martinez, P.R.; Goyanes, A.; Basit, A.W.; Gaisford, S. Fabrication of drug-loaded hydrogels with stereolithographic 3D printing. Int. J. Pharm. 2017, 532, 313–317. [Google Scholar] [CrossRef] [Green Version]
- Schmocker, A.; Khoushabi, A.; Schizas, C.; Bourban, P.-E.; Pioletti, D.P.; Moser, C. Photopolymerizable hydrogels for implants: Monte-Carlo modeling and experimental in vitro validation. J. Biomed. Opt. 2014, 19, 35004. [Google Scholar] [CrossRef] [Green Version]
- Fairbanks, B.D.; Schwartz, M.P.; Bowman, C.N.; Anseth, K.S. Photoinitiated polymerization of PEG-diacrylate with lithium phenyl-2,4,6-trimethylbenzoylphosphinate: Polymerization rate and cytocompatibility. Biomaterials 2009, 30, 6702–6707. [Google Scholar] [CrossRef] [Green Version]
- Hiller, J.D.; Lipson, H. STL 2.0: A proposal for a universal multi-material Additive Manufacturing File format. In Proceedings of the 20th Annual International Solid Freeform Fabrication Symposium (SFF), Austin, TX, USA, 3–5 August 2009; Volume 1, pp. 266–278. [Google Scholar]
- Nguyen, Q.T.; Hwang, Y.; Chen, A.C.; Varghese, S.; Sah, R.L. Cartilage-like mechanical properties of poly (ethylene glycol)-diacrylate hydrogels. Biomaterials 2012, 33, 6682–6690. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Ma, M.; Wang, J.; Zhang, W.; Lu, W.; Gao, Y.; Zhang, B.; Guo, Y. Development of a photo-crosslinking, biodegradable GelMA/PEGDA hydrogel for guided bone regeneration materials. Materials 2018, 11, 1345. [Google Scholar] [CrossRef] [Green Version]
- Lim, K.S.; Schon, B.S.; Mekhileri, N.V.; Brown, G.C.J.; Chia, C.M.; Prabakar, S.; Hooper, G.J.; Woodfield, T.B.F. New Visible-Light Photoinitiating System for Improved Print Fidelity in Gelatin-Based Bioinks. ACS Biomater. Sci. Eng. 2016, 2, 1752–1762. [Google Scholar] [CrossRef]
- Knowlton, S.; Yenilmez, B.; Anand, S.; Tasoglu, S. Photocrosslinking-based bioprinting: Examining crosslinking schemes. Bioprinting 2017, 5, 10–18. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, D.; Alexander, P.G.; Yang, G.; Tan, J.; Cheng, A.W.-M.; Tuan, R.S. Application of visible light-based projection stereolithography for live cell-scaffold fabrication with designed architecture. Biomaterials 2013, 34, 331–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debroy, D.; Oakey, J.; Li, D. Interfacially-mediated oxygen inhibition for precise and continuous poly(ethylene glycol) diacrylate (PEGDA) particle fabrication. J. Colloid Interface Sci. 2018, 510, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Holmes, R.; Yang, X.-B.; Dunne, A.; Florea, L.; Wood, D.; Tronci, G. Thiol-Ene Photo-Click Collagen-PEG Hydrogels: Impact of Water-Soluble Photoinitiators on Cell Viability, Gelation Kinetics and Rheological Properties. Polymers 2017, 9, 226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavallo, A.; Madaghiele, M.; Masullo, U.; Lionetto, M.G.; Sannino, A. Photo-crosslinked poly(ethylene glycol) diacrylate (PEGDA) hydrogels from low molecular weight prepolymer: Swelling and permeation studies. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Machekposhti, S.A.; Mohaved, S.; Narayan, R.J. Inkjet dispensing technologies: Recent advances for novel drug discovery. Expert Opin. Drug Discov. 2019, 14, 101–113. [Google Scholar] [CrossRef]







| Parameter | Nominal Value | Dry Storage | Storage in Water |
|---|---|---|---|
| sx (mm) | 4 | 4.1 ± 0.14 | 4.85 ± 0.16 |
| sy (mm) | 4 | 3.93 ± 0.036 | 4.66 ± 0.027 |
| sz (mm) | 4 | 4.33 ± 0.038 | 5.14 ± 0.03 |
| Volume (mm3) | 64 | 69.77 | 116.17 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konasch, J.; Riess, A.; Mau, R.; Teske, M.; Rekowska, N.; Eickner, T.; Grabow, N.; Seitz, H. A Novel Hybrid Additive Manufacturing Process for Drug Delivery Systems with Locally Incorporated Drug Depots. Pharmaceutics 2019, 11, 661. https://doi.org/10.3390/pharmaceutics11120661
Konasch J, Riess A, Mau R, Teske M, Rekowska N, Eickner T, Grabow N, Seitz H. A Novel Hybrid Additive Manufacturing Process for Drug Delivery Systems with Locally Incorporated Drug Depots. Pharmaceutics. 2019; 11(12):661. https://doi.org/10.3390/pharmaceutics11120661
Chicago/Turabian StyleKonasch, Jan, Alexander Riess, Robert Mau, Michael Teske, Natalia Rekowska, Thomas Eickner, Niels Grabow, and Hermann Seitz. 2019. "A Novel Hybrid Additive Manufacturing Process for Drug Delivery Systems with Locally Incorporated Drug Depots" Pharmaceutics 11, no. 12: 661. https://doi.org/10.3390/pharmaceutics11120661