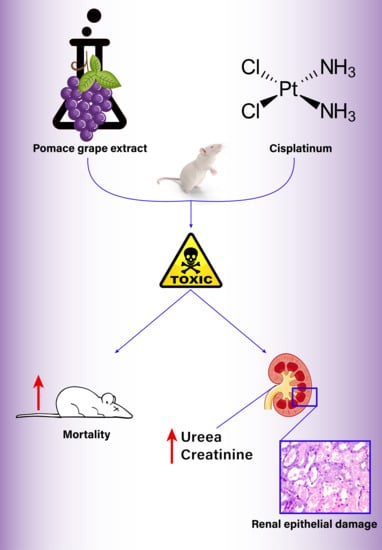
Paradoxical Effect of Grape Pomace Extract on Cisplatin-Induced Acute Kidney Injury in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Grape Extract (GE)
2.3. Experimental Design
2.4. Measurements
2.5. Histopathological Examination
2.6. Statistical Analysis
3. Results
3.1. Mortality
3.2. Urea and Creatinine Levels
3.3. Kidney Histology Examination
3.4. CIS Plasmatic Concentration
3.5. Total Antioxidant Capacity (TAC)
4. Discussion
5. Limitations
- The small number of animals;
- The lack of blood samples from the animals that died during the experiment;
- The missing parameters determined directly from the kidneys;
- The fact that, in natural extracts, it is very unlikely to have the same individual polyphenol concentration and we did not conduct a phytochemical determination of the administered GE homogenate.
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sastry, J.; Kellie, S.J. Severe neurotoxicity, ototoxicity and nephrotoxicity following high-dose cisplatin and amifostine. Pediatr. Hematol. Oncol. 2005, 22, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Arany, I.; Safirstein, R.L. Cisplatin nephrotoxicity. Semin. Nephrol. 2003, 23, 460–464. [Google Scholar] [CrossRef]
- Boulikas, T. Poly(ADP-ribose) synthesis in blocked and damaged cells and its relation to carcinogenesis. Anticancer Res. 1992, 12, 885–898. [Google Scholar] [PubMed]
- Hartmann, J.T.; Fels, L.M.; Knop, S.; Stolt, H.; Kanz, L.; Bokemeyer, C. A randomized trial comparing the nephrotoxicity of cisplatin/ifosfamide-based combination chemotherapy with or without amifostine in patients with solid tumors. Invest. New Drugs 2000, 18, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, J.T.; Lipp, H.-P. Toxicity of platinum compounds. Expert Opin. Pharmacother. 2003, 4, 889–901. [Google Scholar] [CrossRef]
- Cornelison, T.L.; Reed, E. Nephrotoxicity and hydration management for cisplatin, carboplatin, and ormaplatin. Gynecol. Oncol. 1993, 50, 147–158. [Google Scholar] [CrossRef]
- Lehane, D.; Winston, A.; Gray, R.; Daskal, Y. The effect of diuretic pre-treatment on clinical, morphological and ultrastructural cis-platinum induced nephrotoxicity. Int. J. Radiat. Oncol. Biol. Phys. 1979, 5, 1393–1399. [Google Scholar] [CrossRef]
- Al-Sarraf, M.; Fletcher, W.; Oishi, N.; Pugh, R.; Hewlett, J.S.; Balducci, L.; McCracken, J.; Padilla, F. Cisplatin hydration with and without mannitol diuresis in refractory disseminated malignant melanoma: A southwest oncology group study. Cancer Treat. Rep. 1982, 66, 31–35. [Google Scholar]
- Hensley, M.L.; Hagerty, K.L.; Kewalramani, T.; Green, D.M.; Meropol, N.J.; Wasserman, T.H.; Cohen, G.I.; Emami, B.; Gradishar, W.J.; Mitchell, R.B.; et al. American Society of Clinical Oncology 2008 clinical practice guideline update: Use of chemotherapy and radiation therapy protectants. J. Clin. Oncol. 2009, 27, 127–145. [Google Scholar] [CrossRef]
- Castiglione, F.; Dalla Mola, A.; Porcile, G. Protection of normal tissues from radiation and cytotoxic therapy: The development of amifostine. Tumori 1999, 85, 85–91. [Google Scholar]
- Capizzi, R.L. Amifostine reduces the incidence of cumulative nephrotoxicity from cisplatin: Laboratory and clinical aspects. Semin. Oncol. 1999, 26, 72–81. [Google Scholar] [PubMed]
- Hansen, A.S.; Marckmann, P.; Dragsted, L.O.; Finné Nielsen, I.L.; Nielsen, S.E.; Grønbæk, M. Effect of red wine and red grape extract on blood lipids, haemostatic factors, and other risk factors for cardiovascular disease. Eur. J. Clin. Nutr. 2005, 59, 449–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayaprakasha, G.K.; Selvi, T.; Sakariah, K.K. Antibacterial-and-antioxidant-properties-of-GSE.pdf. Food Res. Int. 2003, 36, 117–122. [Google Scholar] [CrossRef]
- Moreno, D.A.; Ilic, N.; Poulev, A.; Brasaemle, D.L.; Fried, S.K.; Raskin, I. Inhibitory effects of grape seed extract on lipases. Nutrition 2003, 19, 876–879. [Google Scholar] [CrossRef]
- Darwish, M.A.; Abo-Youssef, A.M.; Khalaf, M.M.; Abo-Saif, A.A.; Saleh, I.G.; Abdelghany, T.M. Resveratrol influences platinum pharmacokinetics: A novel mechanism in protection against cisplatin-induced nephrotoxicity. Toxicol. Lett. 2018, 290, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Chander, V.; Tirkey, N.; Chopra, K. Resveratrol, a polyphenolic phytoalexin protects against cyclosporine-induced nephrotoxicity through nitric oxide dependent mechanism. Toxicology 2005, 210, 55–64. [Google Scholar] [CrossRef]
- Silan, C.; Uzun, Ö.; Çomunoǧlu, N.Ü.; Gokçen, S.; Bedirhan, S.; Cengiz, M. Gentamicin-induced nephrotoxicity in rats ameliorated and healing effects of resveratrol. Biol. Pharm. Bull. 2007, 30, 79–83. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Xue, J.; Li, Y.; Zhang, W.; Ma, D.; Liu, L.; Zhang, Z. Resveratrol protects against arsenic trioxide-induced nephrotoxicity by facilitating arsenic metabolism and decreasing oxidative stress. Arch. Toxicol. 2013, 87, 1025–1035. [Google Scholar] [CrossRef]
- Do Amaral, C.L.; Francescato, H.D.C.; Coimbra, T.M.; Costa, R.S.; Darin, J.D.A.C.; Antunes, L.M.G.; Bianchi, M.D.L.P. Resveratrol attenuates cisplatin-induced nephrotoxicity in rats. Arch. Toxicol. 2008, 82, 363–370. [Google Scholar] [CrossRef]
- Lopez-Flores, A.; Jurado, R.; Garcia-Lopez, P. A high-performance liquid chromatographic assay for determination of cisplatin in plasma, cancer cell, and tumor samples. J. Pharmacol. Toxicol. Methods 2005, 52, 366–372. [Google Scholar] [CrossRef]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Spitz, D.R.; Phillips, J.W.; Adams, D.T.; Sherman, C.M.; Deen, D.F.; Li, G.C. Cellular resistance to oxidative stress is accompanied by resistance to cisplatin: The significance of increased catalase activity and total glutathione in hydrogen peroxide-resistant fibroblasts. J. Cell. Physiol. 1993, 156, 72–79. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, S.; Finn, S.P.; Cuffe, S.; Richard, D.J.; O’Byrne, K.J.; Barr, M.P. The role of DNA repair pathways in cisplatin resistant lung cancer. Cancer Treat. Rev. 2014, 40, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Prestayko, A.W.; D’Aoust, J.C.; Issell, B.F.; Crooke, S.T. Cisplatin (cis-diamminedichloroplatinum II). Cancer Treat. Rev. 1979, 6, 17–39. [Google Scholar] [CrossRef]
- Florea, A.-M.; Büsselberg, D. Cisplatin as an anti-tumor drug: Cellular mechanisms of activity, drug resistance and induced side effects. Cancers 2011, 3, 1351–1371. [Google Scholar] [CrossRef]
- Dugbartey, G.J.; Peppone, L.J.; de Graaf, I.A.M. An integrative view of cisplatin-induced renal and cardiac toxicities: Molecular mechanisms, current treatment challenges and potential protective measures. Toxicology 2016, 371, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Ramesh, G.; Reeves, W.B. TNF-α mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J. Clin. Investig. 2002, 110, 835–842. [Google Scholar] [CrossRef]
- Demkow, U.; Biatas-Chromiec, B.; Stelmaszczyk-Emmel, A.; Radzikowska, E.; Wiatr, E.; Radwan-Rohrenschef, P.; Szturmowicz, M. The Cardiac Markers and Oxidative Stress Parameters in Advanced Non-Small Cell Lung Cancer Patients Receiving Cisplatin-Based Chemotherapy. EJIFCC 2011, 22, 6–15. [Google Scholar]
- Yang, Z.; Schumaker, L.M.; Egorin, M.J.; Zuhowski, E.G.; Guo, Z.; Cullen, K.J. Cisplatin Preferentially Binds Mitochondrial DNA and Voltage-Dependent Anion Channel Protein in the Mitochondrial Membrane of Head and Neck Squamous Cell Carcinoma: Possible Role in Apoptosis. Clin. Cancer Res. 2006, 12, 5817–5825. [Google Scholar] [CrossRef] [Green Version]
- Jing, X.-B.; Cai, X.-B.; Hu, H.; Chen, S.-Z.; Chen, B.-M.; Cai, J.-Y. Reactive oxygen species and mitochondrial membrane potential are modulated during CDDP-induced apoptosis in EC-109 cells. Biochem. Cell Biol. 2007, 85, 265–271. [Google Scholar] [CrossRef]
- Noori, S.; Mahboob, T. Antioxidant effect of carnosine pretreatment on cisplatin-induced renal oxidative stress in rats. Indian J. Clin. Biochem. 2010, 25, 86–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cetin, R.; Devrim, E.; Kiliçoğlu, B.; Avci, A.; Candir, O.; Durak, I. Cisplatin impairs antioxidant system and causes oxidation in rat kidney tissues: Possible protective roles of natural antioxidant foods. J. Appl. Toxicol. 2006, 26, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Olaku, O.O.; Ojukwu, M.O.; Zia, F.Z.; White, J.D. The Role of Grape Seed Extract in the Treatment of Chemo/Radiotherapy Induced Toxicity: A Systematic Review of Preclinical Studies. Nutr. Cancer 2015, 67, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Trošt, K.; Klančnik, A.; Mozetič Vodopivec, B.; Sternad Lemut, M.; Jug Novšak, K.; Raspor, P.; Smole Možina, S. Polyphenol, antioxidant and antimicrobial potential of six different white and red wine grape processing leftovers. J. Sci. Food Agric. 2016, 96, 4809–4820. [Google Scholar] [CrossRef] [PubMed]
- Balea, Ş.S.; Pârvu, A.E.; Pop, N.; Marín, F.Z.; Pârvu, M. Polyphenolic Compounds, Antioxidant, and Cardioprotective Effects of Pomace Extracts from Fetească Neagră Cultivar. Oxid. Med. Cell. Longev. 2018, 2018, 8194721. [Google Scholar] [CrossRef]
- Ko, J.-L.; Tsai, C.-H.; Liu, T.-C.; Lin, M.-Y.; Lin, H.-L.; Ou, C.-C. Differential effects of grape juice on gastric emptying and renal function from cisplatin-induced acute adverse toxicity. Hum. Exp. Toxicol. 2016, 35, 808–817. [Google Scholar] [CrossRef]
- Wang, C.-Z.; Fishbein, A.; Aung, H.H.; Mehendale, S.R.; Chang, W.-T.; Xie, J.-T.; Li, J.; Yuan, C.-S. Polyphenol Contents in Grape-Seed Extracts Correlate with Antipica Effects in Cisplatin-Treated Rats. J. Altern. Complement. Med. 2005, 11, 1059–1065. [Google Scholar] [CrossRef]
- Fujikura, T.; Yasuda, H.; Iwakura, T.; Tsuji, T.; Anders, H.-J. MDM2 inhibitor ameliorates cisplatin-induced nephropathy via NFκΒ signal inhibition. Pharmacol. Res. Perspect. 2019, 7, e00450. [Google Scholar] [CrossRef] [Green Version]
- Sahu, A.K.; Verma, V.K.; Mutneja, E.; Malik, S.; Nag, T.C.; Dinda, A.K.; Arya, D.S.; Bhatia, J. Mangiferin attenuates cisplatin-induced acute kidney injury in rats mediating modulation of MAPK pathway. Mol. Cell. Biochem. 2019, 452, 141–152. [Google Scholar] [CrossRef]
- Li, X.; Yang, S.; Lv, X.; Sun, H.; Weng, J.; Liang, Y.; Zhou, D. The mechanism of mesna in protection from cisplatin-induced ovarian damage in female rats. J. Gynecol. Oncol. 2013, 24, 177. [Google Scholar] [CrossRef]
- Kociba, R.J.; Sleight, S.D. Acute toxicologic and pathologic effects of cis-diamminedichloroplatinum (NSC-119875) in the male rat. Cancer Chemother. Rep. 1971, 55, 1–8. [Google Scholar] [PubMed]
- Ishida, S.; Lee, J.; Thiele, D.J.; Herskowitz, I. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc. Natl. Acad. Sci. USA 2002, 99, 14298–14302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pabla, N.; Murphy, R.F.; Liu, K.; Dong, Z. The copper transporter Ctr1 contributes to cisplatin uptake by renal tubular cells during cisplatin nephrotoxicity. Am. J. Physiol. Ren. Physiol. 2009, 296, F505–F511. [Google Scholar] [CrossRef] [PubMed]
- Ciarimboli, G.; Ludwig, T.; Lang, D.; Pavenstädt, H.; Koepsell, H.; Piechota, H.-J.; Haier, J.; Jaehde, U.; Zisowsky, J.; Schlatter, E. Cisplatin nephrotoxicity is critically mediated via the human organic cation transporter 2. Am. J. Pathol. 2005, 167, 1477–1484. [Google Scholar] [CrossRef] [Green Version]
- Sprowl, J.A.; Lancaster, C.S.; Pabla, N.; Hermann, E.; Kosloske, A.M.; Gibson, A.A.; Li, L.; Zeeh, D.; Schlatter, E.; Janke, L.J.; et al. Cisplatin-induced renal injury is independently mediated by OCT2 and p53. Clin. Cancer Res. 2014, 20, 4026–4035. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Panichpisal, K.; Kurtzman, N.; Nugent, K. Cisplatin Nephrotoxicity: A Review. Am. J. Med. Sci. 2007, 334, 115–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, Z.-H.; Vanden Hoek, T.L.; Xie, J.; Wojcik, K.; Chan, K.C.; Li, C.-Q.; Hamann, K.; Qin, Y.; Schumacker, P.T.; Becker, L.B.; et al. Grape seed proanthocyanidins induce pro-oxidant toxicity in cardiomyocytes. Cardiovasc. Toxicol. 2003, 3, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-C.; Tsai, H.-I.; Yu, H.-P. Organ-Protective Effects of Red Wine Extract, Resveratrol, in Oxidative Stress-Mediated Reperfusion Injury. Oxid. Med. Cell. Longev. 2015, 2015, 568634. [Google Scholar] [CrossRef]
- Yoshino, M.; Haneda, M.; Naruse, M.; Htay, H.H.; Iwata, S.; Tsubouchi, R.; Murakami, K. Prooxidant action of gallic acid compounds: Copper-dependent strand breaks and the formation of 8-hydroxy-2′-deoxyguanosine in DNA. Toxicol. Vitr. 2002, 16, 705–709. [Google Scholar] [CrossRef]
- Halliwell, B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch. Biochem. Biophys. 2008, 476, 107–112. [Google Scholar] [CrossRef]
- Rechner, A.R.; Kuhnle, G.; Bremner, P.; Hubbard, G.P.; Moore, K.P.; Rice-Evans, C.A. The metabolic fate of dietary polyphenols in humans. Free Radic. Biol. Med. 2002, 33, 220–235. [Google Scholar] [CrossRef]
- Veskoukis, A.S.; Kyparos, A.; Nikolaidis, M.G.; Stagos, D.; Aligiannis, N.; Halabalaki, M.; Chronis, K.; Goutzourelas, N.; Skaltsounis, L.; Kouretas, D. The Antioxidant Effects of a Polyphenol-Rich Grape Pomace Extract in Vitro Do Not Correspond in Vivo Using Exercise as an Oxidant Stimulus. Oxid. Med. Cell. Longev. 2012, 2012, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de la Lastra, C.A.; Villegas, I. Resveratrol as an antioxidant and pro-oxidant agent: Mechanisms and clinical implications. Biochem. Soc. Trans. 2007, 35, 1156–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Group | Mortality (%) |
|---|---|
| Control | 0 |
| GEd1 | 0 |
| GEd2 | 0 |
| CIS | 50 |
| GEd1 + CIS | 62.5 |
| GEd2 + CIS | 75 |
| GEd1 + CIS + GEd1 | 37.5 |
| GEd2 + CIS + GEd2 | 87.5 |
| Group | Score |
|---|---|
| Control | 0 |
| GE1 | 0 |
| GE2 | 0 |
| Cis | 2 |
| GE1 + Cis | 2 |
| GE2 + Cis | 3 |
| GE1 + Cis + GE1 | 1 |
| GE2 + Cis + GE2 | 3 |
| Group | TAC (mmol TR Eq/L) |
|---|---|
| Control | 0.16 |
| GEd1 | 0.18 |
| GEd2 | 0.19 |
| CIS | 0.1 |
| GEd1 + CIS | 0.19 |
| GEd2 + CIS | 0.16 |
| GEd1 + CIS + GEd1 | 0.16 |
| GEd2 + CIS + GEd2 | 0.14 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neag, M.A.; Mitre, C.I.; Mitre, A.O.; Morhan, V.; Catinean, A.; Botan, E.C.; Melincovici, C.S.; Muntean, D.M.; Buzoianu, A.D. Paradoxical Effect of Grape Pomace Extract on Cisplatin-Induced Acute Kidney Injury in Rats. Pharmaceutics 2019, 11, 656. https://doi.org/10.3390/pharmaceutics11120656
Neag MA, Mitre CI, Mitre AO, Morhan V, Catinean A, Botan EC, Melincovici CS, Muntean DM, Buzoianu AD. Paradoxical Effect of Grape Pomace Extract on Cisplatin-Induced Acute Kidney Injury in Rats. Pharmaceutics. 2019; 11(12):656. https://doi.org/10.3390/pharmaceutics11120656
Chicago/Turabian StyleNeag, Maria Adriana, Calin Iosif Mitre, Andrei Otto Mitre, Vlad Morhan, Adrian Catinean, Emil Claudiu Botan, Carmen Stanca Melincovici, Dana Maria Muntean, and Anca Dana Buzoianu. 2019. "Paradoxical Effect of Grape Pomace Extract on Cisplatin-Induced Acute Kidney Injury in Rats" Pharmaceutics 11, no. 12: 656. https://doi.org/10.3390/pharmaceutics11120656