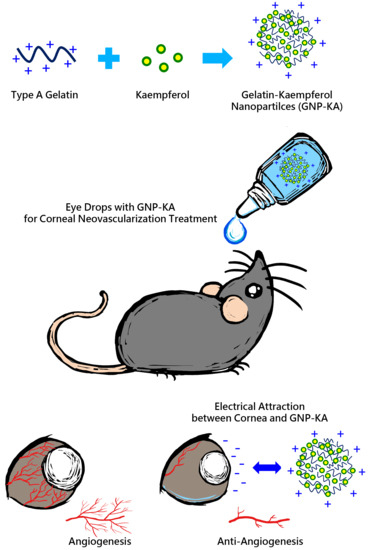
Development of Kaempferol-Loaded Gelatin Nanoparticles for the Treatment of Corneal Neovascularization in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanoparticle Preparation
2.2. Characterization of GNP-KA
2.3. In Vitro Test
2.3.1. Cell Viability Test
2.3.2. Cell Migration Test
2.4. In Vivo Test
2.4.1. Retention of Nanoparticles on the Ocular Surface
2.4.2. Corneal NV Mice Model Establishment
2.4.3. Angiogenetic Factors Examination from Cornea Lysate
2.5. Statistical Analysis
3. Results and Discussion
3.1. Optimal Parameters for GNP- KA Preparation
3.2. Characterization of GNP-KA by TEM and FT-IR
3.3. HUVECs Cell Viability and Migration Capacity Influenced by GNP-KA
3.4. Nanoparticles Retain on the Ocular Surface
3.5. The Effectiveness of the GNP-KA for Treating Corneal Neovascularization
3.6. Histological Results
3.7. Variation of Angiogenic Factors in Cornea
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Whitcher, J.P.; Srinivasan, M.; Upadhyay, M.P. Corneal blindness: A global perspective. Bull. World Health Organ. 2001, 79, 214–221. [Google Scholar] [PubMed]
- Chang, J.H.; Garg, N.K.; Lunde, E.; Han, K.Y.; Jain, S.; Azar, D.T. Corneal neovascularization: An anti-VEGF therapy review. Surv. Ophthalmol. 2012, 57, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.T.; Yao, T.C.; Huang, J.L.; Yeh, K.W.; Hwang, Y.S. Clinical manifestations in uveitis patients with and without rheumatic disease in a Chinese population in Taiwan. J. Microbiol. Immunol. Infect. 2017, 50, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Wang, C.C.; Adamis, A.P. Ocular neovascularization: An epidemiologic review. Surv. Ophthalmol. 1998, 43, 245–269. [Google Scholar] [CrossRef]
- Chang, J.H.; Gabison, E.E.; Kato, T.; Azar, D.T. Corneal neovascularization. Curr. Opin. Ophthalmol. 2001, 12, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Ota, I.; Li, X.-Y.; Hu, Y.; Weissa, S.J. Induction of a MT1-MMP and MT2-MMP-dependent basement membrane transmigration program in cancer cells by Snail1. Proc. Natl. Acad. Sci. USA 2009, 106, 20318–20323. [Google Scholar] [CrossRef]
- Philipp, W.; Speicher, L.; Humpel, C. Expression of vascular endothelial growth factor and its receptors in inflamed and vascularized human corneas. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2514–2522. [Google Scholar]
- Butcher, J.M.; Austin, M.; McGalliard, J.; Bourke, R.D. Bilateral cataracts and glaucoma induced by long term use of steroid eye drops. BMJ 1994, 309, 43. [Google Scholar] [CrossRef]
- Dastjerdi, M.H.; Al-Arfaj, K.M.; Nallasamy, N.; Hamrah, P.; Jurkunas, U.V.; Pineda, R.; Pavan-Langston, D.; Dana, R. Topical bevacizumab in the treatment of corneal neovascularization: Results of a prospective, oppen-label, non-comparative study. Arch. Ophthalmol. 2009, 127, 381–389. [Google Scholar] [CrossRef]
- García-Quintanilla, L.; Luaces-Rodríguez, A.; Gil-Martínez, M.; Mondelo-García, C.; Maroñas, O.; Mangas-Sanjuan, V.; González-Barcia, M.; Zarra-Ferro, I.; Aguiar, P.; Otero-Espinar, F.J.; et al. Pharmacokinetics of intravitreal anti-VEGF drugs in age-related macular degeneration. Pharmaceutics 2019, 11, 365. [Google Scholar]
- Martin, D.F.; Maguire, M.G.; Ying, G.S.; Grunwald, J.E.; Fine, S.L.; Jaffe, G.J. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2011, 364, 1897–1908. [Google Scholar] [CrossRef] [PubMed]
- Krizova, D.; Vokrojova, M.; Liehneova, K.; Studeny, P. Treatment of corneal neovascularization using anti-VEGF bevacizumab. J. Ophthalmol. 2014, 2014, 178132. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ma, J. Ocular neovascularization: Implication of endogenous angiogenic inhibitors and potential therapy. Prog. Retin. Eye Res. 2007, 26, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Tolentino, M. Systemic and ocular safety of intravitreal anti-VEGF therapies for ocular neovascular disease. Surv. Ophthalmol. 2011, 56, 95–113. [Google Scholar] [CrossRef]
- Majumdara, S.; Srirangama, R. Potential of the bioflavonoids in the prevention/treatment of ocular disorders. J. Pharm. Pharmacol. 2010, 10, 951–965. [Google Scholar] [CrossRef] [PubMed]
- Vissiennon, C.K.; Nieber, O.K.; Butterweck, V. Route of administration determines the anxiolytic activity of the flavonols kaempferol, quercetin and myricetin—Are they prodrugs? J. Nutr. Biochem. 2012, 23, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Rankin, G.O.; Liu, L.; Daddysman, M.K.; Jiang, B.H.; Chen, Y.C. Kaempferol inhibits angiogenesis and VEGF expression through both HIF dependent and independent pathways in human ovarian cancer cells. Nutr. Cancer 2009, 61, 554–563. [Google Scholar] [CrossRef]
- Chen, A.Y.; Chen, Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef]
- Tseng, C.L.; Hung, Y.J.; Chen, Z.Y.; Fang, H.W.; Chen, K.H. Synergistic effect of artificial tears containing epigallocatechin gallate and hyaluronic acid for the treatment of rabbits with dry eye syndrome. PLoS ONE 2016, 11, e0157982. [Google Scholar] [CrossRef]
- Chang, C.Y.; Wang, M.C.; Miyagawa, T.; Chen, Z.Y.; Lin, F.H.; Chen, K.H.; Liu, G.S.; Tseng, C.L. Preparation of arginine–glycine–aspartic acid-modified biopolymeric nanoparticles containing epigalloccatechin-3-gallate for targeting vascular endothelial cells to inhibit corneal neovascularization. Int. J. Nanomed. 2017, 12, 279–294. [Google Scholar] [CrossRef]
- Liang, F.; Han, Y.; Gao, H.; Xin, S.; Chen, S.; Wang, N.; Qin, W.; Zhong, H.; Lin, S.; Yao, X.; et al. Kaempferol identified by zebrafish assay and fine fractionations strategy from Dysosma versipellis inhibits angiogenesis through VEGF and FGF pathways. Sci. Rep. 2015, 5, 14468. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Cholkar, K.; Agrahari, V.; Mitra, A.K. Ocular drug delivery systems: An overview. World J. Pharmacol. 2013, 2, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular drug delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Bachu, R.D.; Chowdhury, P.; Al-Saedi, Z.H.F.; Karla, P.K.; Boddu, S.H.S. Ocular drug delivery barriers—Role of nanocarriers in the treatment of anterior segment ocular diseases. Pharmaceutics 2018, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.C. Ocular drug delivery conventional ocular formulations. Adv. Drug Deliv. Rev. 1995, 16, 39–43. [Google Scholar] [CrossRef]
- Tsai, C.H.; Wang, P.Y.; Lin, I.C.; Huang, H.; Liu, G.S.; Tseng, C.L. Ocular drug delivery: Role of degradable polymeric nanocarriers for ophthalmic application. Int. J. Mol. Sci. 2018, 19, 2830. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, A.; Kreuter, J. Microspheres and nanoparticles used in ocular delivery systems. Adv. Drug Deliv. Rev. 1995, 16, 61–73. [Google Scholar] [CrossRef]
- Tseng, C.L.; Chen, K.H.; Su, W.Y.; Lee, Y.H.; Wu, C.C.; Lin, F.H. Cationic gelatin nanoparticles for drug delivery to the ocular surface: In vitro and in vivo evaluation. J. Nanomater. 2013, 2013, 7. [Google Scholar] [CrossRef]
- Bhatta, R.S.; Chandasana, H.; Chhonker, Y.S.; Rathi, C.; Kumar, D.; Mitra, K.; Shukla, P.K. Mucoadhesive nanoparticles for prolonged ocular delivery of natamycin: In vitro and pharmacokinetics studies. Int. J. Pharm. 2012, 432, 105–112. [Google Scholar] [CrossRef]
- Lee, J.E.; Kim, K.L.; Kim, D.; Yeo, Y.; Han, H.; Kim, M.G.; Kim, S.H.; Kim, H.; Jeong, J.H.; Suh, W. Apatinib-loaded nanoparticles suppress vascular endothelial growth factor-induced angiogenesis and experimental corneal neovascularization. Int. J. Nanomed. 2017, 12, 4813–4822. [Google Scholar] [CrossRef]
- Kalam, M.A. The potential application of hyaluronic acid coated chitosan nanoparticles in ocular delivery of dexamethasone. Int. J. Biol. Macromol. 2016, 89, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Coester, C.J.; Langer, K.; Briesen, H.V.; Kreuter, J. Gelatin nanoparticles by two step desolvation—A new preparation method, surface modifications and cell uptake. J. Microencapsul. 2000, 17, 187–193. [Google Scholar] [PubMed]
- Chan, E.C.; van Wijngaarden, P.; Chan, E.; Ngo, D.; Wang, J.-H.; Peshavariya, H.M.; Dusting, G.J.; Liu, G.-S. NADPH oxidase 2 plays a role in experimental corneal neovascularisation. Clin. Sci. 2016, 130, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Halder, A.; Das, S.; Bera, T.; Mukherjee, A. Rapid synthesis for monodispersed gold nanoparticles in kaempferol and anti-leishmanial efficacy against wild and drug resistant strains. RSC Adv. 2017, 7, 14159–14167. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.; Rankin, G.O.; Juliano, N.; Jiang, B.-H.; Chen, Y.C. Kaempferol inhibits VEGF expression and in vitro angiogenesis through a novel ERK-NFκB-cMyc-p21 pathway. Food Chem. 2012, 130, 321–328. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.; Jiang, B.; Li, B.; Li, Z.; Jiang, B.-H.; Chen, Y.C. Kaempferol nanoparticles achieve strong and selective inhibition of ovarian cancer cell viability. Int. J. Nanomed. 2012, 7, 3951–3959. [Google Scholar]
- Du, W.; An, Y.; He, X.; Zhang, D.; He, W. Protection of Kaempferol on oxidative stress-induced retinal pigment epithelial cell damage. Oxid. Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef]
- Lin, C.; Wu, F.; Zheng, T.; Wang, X.; Chen, Y.; Wu, X. Kaempferol attenuates retinal ganglion cell death by suppressing NLRP1/NLRP3 inflammasomes and caspase-8 via JNK and NF-κB pathways in acute glaucoma. Eye 2019, 33, 777–784. [Google Scholar] [CrossRef]
- Ilk, S.; Saglam, N.; Özgen, M. Kaempferol loaded lecithin/chitosan nanoparticles: Preparation, characterization, and their potential applications as a sustainable antifungal agent. Artif. Cells Nanomed. B 2017, 45, 907–916. [Google Scholar] [CrossRef] [Green Version]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Gondal, T.A.; Saeed, F.; Imran, A.; Shahbaz, M.; Fokou, P.V.T.; Arshad, M.U.; Khan, H.; et al. Kaempferol: A key emphasis to its anticancer potential. Molecules 2019, 24, 2227. [Google Scholar] [CrossRef] [Green Version]












| GA Concn. | Size (nm) | Zeta (mV) | PDI | EE (%) | LR (%) |
|---|---|---|---|---|---|
| 0.1 (v/v %) | 281 ± 16 | +21.5 ± 0.5 | 0.199 ± 0.020 | 85 ± 10 | 2.1 ± 0.3 |
| 0.4 (v/v %) | 148 ± 10 | +24.4 ± 1.9 | 0.203 ± 0.032 | 96 ± 2 | 2.4 ± 0.1 |
| Crosslinking Time | Size (nm) | Zeta (mV) | PDI | EE (%) | LR (%) |
|---|---|---|---|---|---|
| 1 h | 138 ± 3 | +22.8 ± 0.7 | 0.330 ± 0.032 | 90 ± 2 | 2.2 ± 0.1 |
| 3 h | 149 ± 10 | +24.4 ± 1.9 | 0.203 ± 0.031 | 96 ± 2 | 2.4 ± 0.1 |
| 16 h | 90 ± 8 | +21.4 ± 0.1 | 0.275 ± 0.010 | 98 ± 1 | 2.4 ± 0.0 |
| Gelatin Concn. | Size (nm) | Zeta (mV) | PDI | EE (%) | LR (%) |
|---|---|---|---|---|---|
| 1% (w/v) * | 133 ± 15 | +26.6 ± 1.4 | 0.211 ± 0.072 | / | / |
| 1% (w/v) | 85 ± 8 | +25.6 ± 2.1 | 0.306 ± 0.051 | 95 ± 1 | 4.6 ± 0.1 |
| 2% (w/v) | 149 ± 10 | +24.4 ± 1.9 | 0.203 ± 0.030 | 96 ± 2 | 2.4 ± 0.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuang, Y.-L.; Fang, H.-W.; Ajitsaria, A.; Chen, K.-H.; Su, C.-Y.; Liu, G.-S.; Tseng, C.-L. Development of Kaempferol-Loaded Gelatin Nanoparticles for the Treatment of Corneal Neovascularization in Mice. Pharmaceutics 2019, 11, 635. https://doi.org/10.3390/pharmaceutics11120635
Chuang Y-L, Fang H-W, Ajitsaria A, Chen K-H, Su C-Y, Liu G-S, Tseng C-L. Development of Kaempferol-Loaded Gelatin Nanoparticles for the Treatment of Corneal Neovascularization in Mice. Pharmaceutics. 2019; 11(12):635. https://doi.org/10.3390/pharmaceutics11120635
Chicago/Turabian StyleChuang, Yu-Lun, Hsu-Wei Fang, Aditya Ajitsaria, Ko-Hua Chen, Chen-Ying Su, Guei-Sheung Liu, and Ching-Li Tseng. 2019. "Development of Kaempferol-Loaded Gelatin Nanoparticles for the Treatment of Corneal Neovascularization in Mice" Pharmaceutics 11, no. 12: 635. https://doi.org/10.3390/pharmaceutics11120635