Development of an Oral Compound Pickering Emulsion Composed of Nanocrystals of Poorly Soluble Ingredient and Volatile Oils from Traditional Chinese Medicine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
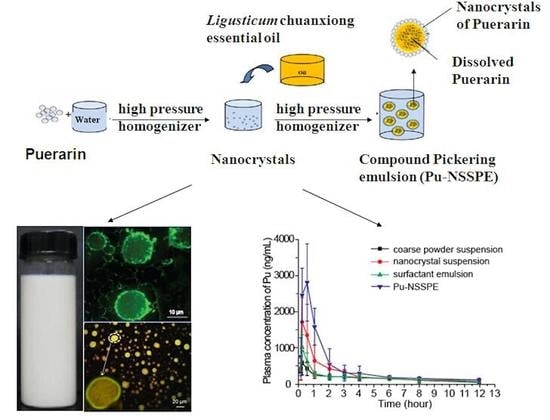
2.2. Preparation and Characterization of Puerarin Nanocrystal Suspension (Pu-NCS)
2.3. Preparation of Pu-NSSPE
2.4. Stability of Pu-NSSPE
2.5. Microstructure Characterization of Pu-NSSPE
2.5.1. Scanning Electron Microscopy
2.5.2. Fluorescence Microscope
2.5.3. Confocal Laser Scanning Microscope
2.5.4. Differential Scanning Calorimetry
2.6. Pharmacokinetic Study in Rats
2.6.1. Preparation of Control Samples
2.6.2. Drug Administration and Sampling
2.6.3. Puerarin Blood Concentration Analysis
2.7. Data and Statistical Analyses
3. Results and Discussions
3.1. Characterization of Pu-NCS
3.2. Stability of Pu-NSSPE
3.3. Microstructure Characterization of Pu-NSSPE
3.4. Pharmacokinetic Study in Rats
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zhou, B.X.; Wu, S.X.; Sun, M.Y.; Hu, R.X. Research progress on co-crystal of insoluble active ingredients of Chinese material medica. Chin. Trad. Herbal Drugs 2016, 47, 336–343. [Google Scholar]
- Zhang, X.W.; Xing, H.J.; Zhao, Y.; Ma, Z.G. Pharmaceutical dispersion techniques for dissolution and bioavailability enhancement of poorly water-soluble drugs. Pharmaceutics 2018, 23, 74–106. [Google Scholar] [CrossRef] [PubMed]
- Ting, J.M.; Porter, W.W.; Mecca, J.M.; Bates, F.S.; Reineke, T.M. Advances in Polymer Design for Enhancing Oral Drug Solubility and Delivery. Bioconjugate Chem. 2018, 29, 939–952. [Google Scholar] [CrossRef] [PubMed]
- Kotta, S.; Khan, A.W.; Pramod, K.; Ansari, S.H.; Sharma, R.K.; Ali, J. Exploring oral nanoemulsions for bioavailability enhancement of poorly water-soluble drugs. Expert Opin. Drug Del. 2012, 9, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Rayner, M.; Marku, D.; Eriksson, M.; Sjoo, M.; Dejmek, P.; Wahlgren, M. Biomass-based particles for the formulation of Pickering type emulsions in food and topical applications. Colloids Surf. A 2014, 458, 48–62. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Fang, Z.W.; Chen, X.; Zhang, W.W.; Xie, Y.M.; Chen, Y.H.; Liu, Z.G.; Yuan, W.E. An overview of Pickering emulsions: solid-particle materials, classification, morphology, and applications. Front. Pharmacol. 2017, 23, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Marto, J.; Ascenso, A.; Simoes, S.; Almeida, A.J.; Ribeiro, H.M. Pickering emulsions: Challenges and opportunities in topical delivery. Expert Opin. Drug Del. 2106, 13, 1093–1107. [Google Scholar] [CrossRef] [PubMed]
- Frelichowska, J.; Bolzinger, M.A.; Pelletier, J.; Valour, J.P.; Chevalier, Y. Topical delivery of lipophilic drugs from o/w Pickering emulsions. Int. J. Pharm. 2009, 371, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Elmotasem, H.; Farag, H.K.; Salama, A.A.A. In vitro and in vivo evaluation of an oral sustained release hepatoprotective caffeine loaded w/o Pickering emulsion formula-Containing wheat germ oil and stabilized by magnesium oxide nanoparticles. Int. J. Pharm. 2108, 547, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.; Liu, C.; Zhang, J.; Wang, F.; Wang, J.R.; Zhang, J.F. A new drug nanocrystal self-stabilized Pickering emulsion for oral delivery of silybin. Eur. J. Pharm. Sci. 2017, 96, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, Y.; Bolzinger, M.A. Emulsions stabilized with solid nanoparticles: Pickering emulsions. Colloids Surf. A 2013, 439, 23–34. [Google Scholar] [CrossRef]
- Xiao, J.; Li, Y.Q.; Huang, Q.R. Recent advances on food-grade particles stabilized Pickering emulsions: Fabrication, characterization and research trends. Trends Food Sci. Techol. 2016, 55, 48–60. [Google Scholar] [CrossRef] [Green Version]
- Wei, S.Y.; Chen, Y.; Xu, X.Y. Progress on the pharmacological research of puerarin: A review. Chin. J. Nat. Med. 2014, 12, 407–414. [Google Scholar] [CrossRef]
- Liu, Y.; Xue, Q.; Li, X.; Zhang, J.F.; Fu, Z.F.; Feng, B.B.; Chen, Y.; Xu, X.Y. Amelioration of Stroke-Induced Neurological Deficiency by Lyophilized Powder of Catapol and Puerarin. Int. J. Biol. Sci. 2014, 10, 448–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.F.; Mei, Z.G.; Fu, Y.; Yang, S.B.; Zhang, S.Z.; Huang, W.F.; Xiong, L.; Zhou, H.J.; Tao, W.; Feng, Z.T. Puerarin protects rat brain against ischemia/reperfusion injury by suppressing autophagy via the AMPK-mTOR-ULK1 signaling pathway. Neural Regener. Res. 2018, 13, 989–998. [Google Scholar]
- Yi, T.; Tang, D.D.; Wang, F.; Zhang, J.Q.; Zhang, J.; Wang, J.R.; Xu, X.Y.; Zhang, J.F. Enhancing both oral bioavailability and brain penetration of puerarin using borneol in combination with preparation technologies. Drug Deliv. 2017, 24, 422–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anukunwithaya, T.; Poo, P.; Hunsakunachai, N.; Rodsiri, R.; Malaivijitnond, S.; Khemawoot, P. Absolute oral bioavailability and disposition kinetics of puerarin in female rats. BMC Pharmacol. Toxicol. 2018, 19, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.N.; Tu, L.X.; Hu, K.L.; Wu, W.; Feng, J.F. The construction of puerarin nanocrystals and its pharmacokinetic and in vivo-in vitro correlation (IVIVC) studies on beagle dog. Colloids Surf. B 2015, 133, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.X.; Yi, Y.N.; Wu, W.; Hu, F.Q.; Hu, K.L.; Feng, J.F. Effects of particle size on the pharmacokinetics of puerarin nanocrystals and microcrystals after oral administration to rat. Int. J. Pharm. 2013, 458, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Zhao, X.H.; Zu, Y.G.; Wang, W.G.; Wu, W.W.; Zhong, C.; Wu, M.F.; Li, Z. Preparation, characterization and bioavailability of oral puerarin nanoparticles by emulsion solvent evaporation method. RSC Adv. 2016, 6, 69889–69901. [Google Scholar] [CrossRef]
- Tang, T.T.; Hu, X.B.; Liao, D.H.; Liu, X.Y.; Xiang, D.X. Mechanisms of microemulsion enhancing the oral bioavailability of puerarin: Comparison between oil-in-water and water-in-oil microemulsions using the single-pass intestinal perfusion method and a chylomicron flow blocking approach. Int. J. Nanomed. 2013, 8, 4415–4426. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, R.R.; Wu, J.; Shen, Q. Characterization and evaluation of self-microemulsifying sustained-release pellet formulation of puerarin for oral delivery. Int. J. Pharm. 2012, 427, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, L.; Zheng, L.Q.; Geng, F. Studies on crystallinity state of puerarin loaded solid lipid nanoparticles prepared by double emulsion method. J. Therm. Anal. Calorim. 2010, 99, 689–693. [Google Scholar] [CrossRef]
- Gu, L.; Wu, Z.H.; Qi, X.L.; He, H.; Ma, X.L.; Chou, X.H.; Wen, X.G.; Zhang, M.; Jiao, F. Polyamidomine dendrimers: An excellent drug carrier for improving the solubility and bioavailability ofpuerarin. Pharm. Dev. Technol. 2013, 18, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.C.; Qiu, B.Y. Problems of surfactants toxicity. Deterg Cosmet. 2005, 28, 22–26. [Google Scholar]
- Du, J.C.; Xie, X.F.; Xiong, L.; Sun, C.; Peng, C. Research progress of chemical constituents and pharmacological activities of essential oil of Ligusticum chuanxiong. China J. Chin. Mat. Med. 2016, 41, 4328–4333. [Google Scholar]
- Qu, D.; Ma, Y.H.; Sun, W.J.; Chen, Y.; Zhou, J.; Liu, C.Y.; Huang, M.M. Microemulsion-based synergistic dual-drug codelivery system for enhanced apoptosis of tumor cells. Int. J. Nanomed. 2015, 10, 1173–1187. [Google Scholar]
- Qu, D.; He, J.J.; Liu, C.Y.; Zhou, J.; Chen, Y. Triterpene-loaded microemulsion using Coix lacryma-jobi seed extract as oil phase for enhanced antitumor efficacy: Preparation and in vivo evaluation. Int. J. Nanomed. 2014, 9, 109–119. [Google Scholar]
- Zhang, H.; Han, T.; Yu, C.H.; Jiang, Y.P.; Peng, C.; Ran, X.; Qin, L.P. Analysis of the chemical composition, acute toxicity and skin sensitivity of essential oil from rhizomes of Ligusticum chuanxiong. J. Ethnopharmacol. 2012, 144, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Sun, Q.A.; Hu, Z.Y.; Liu, H.; Zhou, T.T. Guorong Fan1 Optimization of an accelerated solvent extraction dispersive liquid–liquid microextraction method for the separation and determination of essential oil from Ligusticum chuanxiongHort by gas chromatography with mass spectrometry. J. Sep. Sci. 2015, 38, 3588–3598. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.M.; Lee, Y. Surface modification of zein colloidal particles with sodium caseinate to stabilize oil-in-water pickering emulsion. Food Hydrocolloid. 2016, 56, 292–302. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Wang, S.; Yi, T.; Zhang, J.F. Effects of drug-oil properties on fabrication of drug nanocrystalline self-stabilizied Pickering emulsions. China J. Chin. Mat. Med. 2017, 42, 3739–3746. [Google Scholar]
- Liu, F.; Ou, S.Y.; Tang, C.H. Ca2+-induced soy protein nanoparticles as pickering stabilizers: Fabrication and characterization. Food Hydrocolloid. 2017, 65, 175–186. [Google Scholar] [CrossRef]
- Wu, L.; Liao, Z.H.; Liu, M.Q.; Yin, X.Z.; Li, X.; Wang, M.L.; Lu, X.L.; Lv, N.N.; Singh, V.; He, Z.G. Fabrication of non-spherical Pickering emulsion droplets by cyclodextrins mediated molecular self-assembly. Colloids Surf. A 2016, 490, 163–172. [Google Scholar] [CrossRef]
- Leal-Castaneda, E.J.; Garcia-Tejeda, Y.; Hernandez-Sanchez, H.; Alamilla-Beltran, L.; Tellez-Medina, D.I.; Calderon-Dominguez, G.; Garcia, H.S.; Gutierrez-Lopez, G.F. Pickering emulsions stabilized with native and lauroylated amaranth starch. Food Hydrocolloid. 2018, 80, 177–185. [Google Scholar] [CrossRef]
- Li, G.Z.; Gao, J.S.; Liu, Y.M. Fluorescence of Puterrin and Its Application. J. Anal. Sci. 2002, 18, 394–396. [Google Scholar]
- Xiao, J.; Wang, X.A.; Gonzalez, A.J.P.; Huang, Q.R. Kafirin nanoparticles-stabilized Pickering emulsions: Microstructure and rheological behavior. Food Hydrocolloid. 2016, 54, 30–39. [Google Scholar] [CrossRef]
- Kruglyakov, P.M.; Nushtayeva, A.V. Phase inversion in emulsions stabilised by solid particles. Adv. Colloid Interfac Sci. 2004, 108, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Marina, P.A.F.; Delcheva, I.; Beattie, D.A. Multi-modal stabilisation of emulsions using a combination of hydrophilic particles and an amino acid. Colloids Surf. A 2018, 538, 765–773. [Google Scholar] [CrossRef]
- Li, H.W.; Dong, L.; Liu, Y.; Wang, G.P.; Wang, G.; Qiao, Y.J. Biopharmaceutics classification of puerarin and comparison of perfusion approaches in rats. Int. J. Pharm. 2014, 466, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.; Simovic, S.; Davey, A.K.; Rades, T.; Prestidge, C.A. Silica-lipid hybrid (SLH) microcapsules: A novel oral delivery system for poorly soluble drugs. J. Control. Release 2009, 134, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Simovic, S.; Hui, H.; Song, Y.M.; Davey, A.K.; Rades, T.; Prestidge, C.A. An oral delivery system for indomethicin engineered from cationic lipid emulsions and silica nanoparticles. J. Control. Release 2010, 143, 367–373. [Google Scholar] [CrossRef] [PubMed]




 ), 25 (
), 25 (  ), and 40 (
), and 40 (  ) °C.
) °C.
 ), 25 (
), 25 (  ), and 40 (
), and 40 (  ) °C.
) °C.





| Parameter | Pu-CPS | Pu-NCS | Pu-SE | Pu-NSSPE |
|---|---|---|---|---|
| AUC0–t/ng·mL–1·h | 1903.05 ± 272.12 | 3203.11 ± 1021.48 # | 2233.01 ± 341.56 | 4994.22 ± 1650.83 # #,Δ,** |
| Tmax/h | 0.22 ± 0.14 | 0.22 ± 0.14 | 0.17 ± 0.00 | 0.33 ± 0.18 * |
| Cmax/ng·mL–1 | 634.17 ± 292.77 | 1881.44 ± 731.84 # # | 1021.38 ± 359.49 Δ | 3226.14 ± 726.64 # #,ΔΔ,** |
| t1/2/h | 3.14 ± 1.20 | 5.48 ± 1.89 # | 5.32 ± 1.56 # # | 5.57 ± 1.16 # # |
| MRT/h | 3.82 ± 0.98 | 3.16 ± 1.10 # # | 3.86 ± 0.60 | 2.09 ± 0.60 # #,Δ,** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhang, J.; Wang, S.; Yi, T. Development of an Oral Compound Pickering Emulsion Composed of Nanocrystals of Poorly Soluble Ingredient and Volatile Oils from Traditional Chinese Medicine. Pharmaceutics 2018, 10, 170. https://doi.org/10.3390/pharmaceutics10040170
Zhang J, Zhang J, Wang S, Yi T. Development of an Oral Compound Pickering Emulsion Composed of Nanocrystals of Poorly Soluble Ingredient and Volatile Oils from Traditional Chinese Medicine. Pharmaceutics. 2018; 10(4):170. https://doi.org/10.3390/pharmaceutics10040170
Chicago/Turabian StyleZhang, Jifen, Jiao Zhang, Shuai Wang, and Tao Yi. 2018. "Development of an Oral Compound Pickering Emulsion Composed of Nanocrystals of Poorly Soluble Ingredient and Volatile Oils from Traditional Chinese Medicine" Pharmaceutics 10, no. 4: 170. https://doi.org/10.3390/pharmaceutics10040170