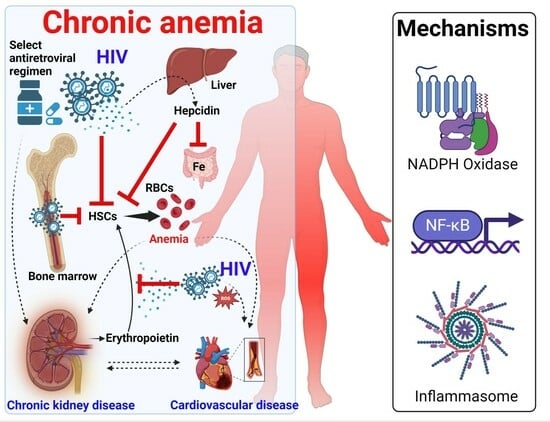
Mechanisms and Cardiorenal Complications of Chronic Anemia in People with HIV
Abstract
:1. Introduction
2. Burden of Anemia in People with HIV
2.1. Prevalence of Anemia
2.2. Duration of ART and Anemia Risk
3. Underlying Mechanisms of Chronic Anemia in HIV
3.1. HIV Reactivation and Infection of Hematopoietic Stem Cells Mediated by the TNF-α-Dependent NF-κB Pathway
3.2. Other Mechanisms of HIV Impairment of Hematopoietic Stem Cells beyond the TNF-α-Dependent NF-κB Pathway
3.3. Effect of HIV-1 Infection on Erythropoietin Production
4. The Inflammatory Milieu in HIV Contributes to Chronic Anemia and Associated Adverse Outcomes
5. Chronic Anemia in HIV Is Associated with Chronic Kidney Disease and Cardiovascular Disease
5.1. Chronic Anemia and Chronic Kidney Disease in HIV
5.2. Chronic Inflammation Increases the Risk for Cardiovascular Disease in HIV
6. Therapeutic Strategies to Control Anemia in HIV
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- UNAIDS Global HIV & AIDS Statistics—Fact Sheet. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 30 September 2023).
- Bhardwaj, S.; Almaeen, A.; Ahmed Wani, F.; Thirunavukkarasu, A. Hematologic Derangements in HIV/AIDS Patients and Their Relationship with the CD4 Counts: A Cross-Sectional Study. Int. J. Clin. Exp. Pathol. 2020, 13, 756–763. [Google Scholar] [PubMed]
- Cao, G.; Wang, Y.; Wu, Y.; Jing, W.; Liu, J.; Liu, M. Prevalence of Anemia among People Living with HIV: A Systematic Review and Meta-Analysis. eClinicalMedicine 2022, 44, 101283. [Google Scholar] [CrossRef] [PubMed]
- Haider, B.A.; Spiegelman, D.; Hertzmark, E.; Sando, D.; Duggan, C.; Makubi, A.; Sudfeld, C.; Aris, E.; Chalamilla, G.E.; Fawzi, W.W. Anemia, Iron Deficiency, and Iron Supplementation in Relation to Mortality among HIV-Infected Patients Receiving Highly Active Antiretroviral Therapy in Tanzania. Am. J. Trop. Med. Hyg. 2019, 100, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Obirikorang, C.; Issahaku, R.G.; Osakunor, D.N.M.; Osei-Yeboah, J. Anaemia and Iron Homeostasis in a Cohort of HIV-Infected Patients: A Cross-Sectional Study in Ghana. AIDS Res. Treat. 2016, 2016, e1623094. [Google Scholar] [CrossRef] [PubMed]
- Cappellini, M.D.; Motta, I. Anemia in Clinical Practice-Definition and Classification: Does Hemoglobin Change With Aging? Semin. Hematol. 2015, 52, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Harding, B.N.; Whitney, B.M.; Nance, R.M.; Ruderman, S.A.; Crane, H.M.; Burkholder, G.; Moore, R.D.; Mathews, W.C.; Eron, J.J.; Hunt, P.W.; et al. Anemia Risk Factors among People Living with HIV across the United States in the Current Treatment Era: A Clinical Cohort Study. BMC Infect. Dis. 2020, 20, 238. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Gummuluru, S. HIV-1 Persistence and Chronic Induction of Innate Immune Responses in Macrophages. Viruses 2020, 12, 711. [Google Scholar] [CrossRef] [PubMed]
- Sereti, I.; Estes, J.D.; Thompson, W.L.; Morcock, D.R.; Fischl, M.A.; Croughs, T.; Beq, S.; de Micheaux, S.L.; Yao, M.D.; Ober, A.; et al. Decreases in Colonic and Systemic Inflammation in Chronic HIV Infection after IL-7 Administration. PLOS Pathog. 2014, 10, e1003890. [Google Scholar] [CrossRef] [PubMed]
- Zicari, S.; Sessa, L.; Cotugno, N.; Ruggiero, A.; Morrocchi, E.; Concato, C.; Rocca, S.; Zangari, P.; Manno, E.C.; Palma, P. Immune Activation, Inflammation, and Non-AIDS Co-Morbidities in HIV-Infected Patients under Long-Term ART. Viruses 2019, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Ta, T.M.; Malik, S.; Anderson, E.M.; Jones, A.D.; Perchik, J.; Freylikh, M.; Sardo, L.; Klase, Z.A.; Izumi, T. Insights Into Persistent HIV-1 Infection and Functional Cure: Novel Capabilities and Strategies. Front. Microbiol. 2022, 13, 862270. [Google Scholar] [CrossRef]
- Megha, K.B.; Joseph, X.; Akhil, V.; Mohanan, P.V. Cascade of Immune Mechanism and Consequences of Inflammatory Disorders. Phytomedicine 2021, 91, 153712. [Google Scholar] [CrossRef] [PubMed]
- Borges, Á.H.; Weitz, J.I.; Collins, G.; Baker, J.V.; Lévy, Y.; Davey, R.T.; Phillips, A.N.; Neaton, J.D.; Lundgren, J.D.; Deeks, S.G.; et al. Markers of Inflammation and Activation of Coagulation Are Associated with Anaemia in Antiretroviral-Treated HIV Disease. Acquir. Immune Defic. Syndr. 2014, 28, 1791–1796. [Google Scholar] [CrossRef] [PubMed]
- Minchella, P.A.; Armitage, A.E.; Darboe, B.; Jallow, M.W.; Drakesmith, H.; Jaye, A.; Prentice, A.M.; McDermid, J.M. Elevated Hepcidin Is Part of a Complex Relation That Links Mortality with Iron Homeostasis and Anemia in Men and Women with HIV Infection123. J. Nutr. 2015, 145, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Suega, K.; Widiana, G.R. Predicting hepcidin level using inflammation markers and iron indicators in patients with anemia of chronic disease. Rev. Bras. Hematol. Hemoter. 2019, 41, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Hepcidin. Rinsho Ketsueki 2016, 57, 1913–1917. [Google Scholar] [CrossRef] [PubMed]
- Abioye, A.I.; Andersen, C.T.; Sudfeld, C.R.; Fawzi, W.W. Anemia, Iron Status, and HIV: A Systematic Review of the Evidence. Adv. Nutr. 2020, 11, 1334–1363. [Google Scholar] [CrossRef] [PubMed]
- Lanser, L.; Fuchs, D.; Kurz, K.; Weiss, G. Physiology and Inflammation Driven Pathophysiology of Iron Homeostasis-Mechanistic Insights into Anemia of Inflammation and Its Treatment. Nutrients 2021, 13, 3732. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Ganz, T.; Goodnough, L.T. Anemia of Inflammation. Blood 2019, 133, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.R.; Zauli, G. Effect of Human Immunodeficiency Virus Infection on Haematopoiesis. Baillieres Clin. Haematol. 1995, 8, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, T. Hematopoietic Stem/Progenitor Cells and the Pathogenesis of HIV/AIDS. Front. Cell Infect. Microbiol. 2020, 10, 60. [Google Scholar] [CrossRef] [PubMed]
- Alexaki, A.; Wigdahl, B. HIV-1 Infection of Bone Marrow Hematopoietic Progenitor Cells and Their Role in Trafficking and Viral Dissemination. PLoS Pathog. 2008, 4, e1000215. [Google Scholar] [CrossRef] [PubMed]
- Gibellini, D.; Clò, A.; Morini, S.; Miserocchi, A.; Ponti, C.; Re, M.C. Effects of Human Immunodeficiency Virus on the Erythrocyte and Megakaryocyte Lineages. World J. Virol. 2013, 2, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, T. HIV Impacts CD34+ Progenitors Involved in T-Cell Differentiation During Coculture With Mouse Stromal OP9-DL1 Cells. Front. Immunol. 2019, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.L.; Chen, Y.H.; Liu, Y.M.; Yuan, J.J.; Lin, J.; Huang, A.Q.; Ye, H.H. Prevalence and Risk Factors of Anaemia in Hospitalised HIV-Infected Patients in Southeast China: A Retrospective Study. Epidemiol. Infect. 2019, 147, e81. [Google Scholar] [CrossRef]
- Sah, S.K.; Dahal, P.; Tamang, G.B.; Mandal, D.K.; Shah, R.; Pun, S.B. Prevalence and Predictors of Anemia in HIV-Infected Persons in Nepal. HIV/AIDS-Res. Palliat. Care 2020, 12, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Berhane, Y.; Haile, D.; Tolessa, T. Anemia in HIV/AIDS Patients on Antiretroviral Treatment at Ayder Specialized Hospital, Mekele, Ethiopia: A Case-Control Study. J. Blood Med. 2020, 11, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Ageru, T.A.; Koyra, M.M.; Gidebo, K.D.; Abiso, T.L. Anemia and Its Associated Factors among Adult People Living with Human Immunodeficiency Virus at Wolaita Sodo University Teaching Referral Hospital. PLoS ONE 2019, 14, e0221853. [Google Scholar] [CrossRef] [PubMed]
- Dobe, I.; Manafe, N.; Majid, N.; Zimba, I.; Manuel, B.; Mocumbi, A. Patterns of Cardiovascular Risk and Disease in HIV-Positive Adults on Anti-Retroviral Therapy in Mozambique. Cardiovasc. J. Afr. 2020, 31, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Ezeamama, A.E.; Sikorskii, A.; Bajwa, R.K.; Tuke, R.; Kyeyune, R.B.; Fenton, J.I.; Guwatudde, D.; Fawzi, W.W. Evolution of Anemia Types During Antiretroviral Therapy—Implications for Treatment Outcomes and Quality of Life Among HIV-Infected Adults. Nutrients 2019, 11, 755. [Google Scholar] [CrossRef] [PubMed]
- Kerkhoff, A.D.; Wood, R.; Cobelens, F.G.; Gupta-Wright, A.; Bekker, L.-G.; Lawn, S.D. The Predictive Value of Current Haemoglobin Levels for Incident Tuberculosis and/or Mortality during Long-Term Antiretroviral Therapy in South Africa: A Cohort Study. BMC Med. 2015, 13, 70. [Google Scholar] [CrossRef] [PubMed]
- Tamir, Z.; Alemu, J.; Tsegaye, A. Anemia among HIV Infected Individuals Taking ART with and without Zidovudine at Addis Ababa, Ethiopia. Ethiop. J. Health Sci. 2018, 28, 73. [Google Scholar] [CrossRef] [PubMed]
- Firnhaber, C.; Smeaton, L.; Saukila, N.; Flanigan, T.; Gangakhedkar, R.; Kumwenda, J.; La Rosa, A.; Kumarasamy, N.; De Gruttola, V.; Hakim, J.G.; et al. Comparisons of Anemia, Thrombocytopenia, and Neutropenia at Initiation of HIV Antiretroviral Therapy in Africa, Asia, and the Americas. Int. J. Infect. Dis. 2010, 14, e1088–e1092. [Google Scholar] [CrossRef] [PubMed]
- Katemba, C.; Muzoora, C.; Muwanguzi, E.; Mwambi, B.; Atuhairwe, C.; Taremwa, I.M. Hematological Abnormalities in HIV-Antiretroviral Therapy Naïve Clients as Seen at an Immune Suppression Syndrome Clinic at Mbarara Regional Referral Hospital, Southwestern Uganda. J. Blood Med. 2018, 9, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Johannessen, A.; Naman, E.; Gundersen, S.G.; Bruun, J.N. Antiretroviral Treatment Reverses HIV-Associated Anemia in Rural Tanzania. BMC Infect. Dis. 2011, 11, 190. [Google Scholar] [CrossRef] [PubMed]
- Petraro, P.; Duggan, C.; Spiegelman, D.; Hertzmark, E.; Makubi, A.; Chalamilla, G.; Siril, H.; Sando, D.; Aboud, S.; Fawzi, W.W. Determinants of Anemia among Human Immunodeficiency Virus-Positive Adults at Care and Treatment Clinics in Dar Es Salaam, Tanzania. Am. J. Trop. Med. Hyg. 2016, 94, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Meidani, M.; Rezaei, F.; Maracy, M.R.; Avijgan, M.; Tayeri, K. Prevalence, Severity, and Related Factors of Anemia in HIV/AIDS Patients. J. Res. Med. Sci. 2012, 17, 138–142. [Google Scholar] [PubMed]
- Ciccacci, F.; Orlando, S.; Sagno, J.B.; Kamponda, M.; Gondwe, J.; Lunghi, R.; Marazzi, M.C.; Palombi, L. Evaluation of Nutritional Conditions, Haemoglobin Levels, Retention in Care and Viral Suppression in a Cohort of HIV-Infected Malawian Adolescents Undergoing a One-Year Tailored Intervention within the Diseases Relief through Excellence and Advanced Means Programme. South. Afr. J. Child. Health 2020, 14, 228–232. [Google Scholar]
- Belay, A.S.; Genie, Y.D.; Kebede, B.F.; Kassie, A.; Molla, A. Original Research: Time to Detection of Anaemia and Its Predictors among Women of Reproductive-Age Living with HIV/AIDS Initiating ART at Public Hospitals, Southwest Ethiopia: A Multicentre Retrospective Follow-up Study. BMJ Open 2022, 12, e059934. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Zhao, Y.; Gan, X.; Zhao, D.; Wu, Y.; Dou, Z.; Ma, Y. The Burden of Anemia among Chinese HIV-Infected Patients Following the Initiation of Antiretroviral Therapy in the Treat-All Era: A Nationwide Cohort Study. BMC Infect. Dis. 2023, 23, 704. [Google Scholar] [CrossRef] [PubMed]
- Manaye, Y.; Asrat, A.; Mengesha, E.W. Time to Development of Anemia and Predictors among HIV-Infected Patients Initiating ART at Felege Hiwot Referral Hospital, Northwest Ethiopia: A Retrospective Follow-Up Study. BioMed Res. Int. 2020, 2020, e7901241. [Google Scholar] [CrossRef] [PubMed]
- Ngongondo, M.; Rosenberg, N.E.; Stanley, C.C.; Lim, R.; Ongubo, D.; Broadhurst, R.; Speight, C.; Flick, R.; Tembo, P.; Hosseinpour, M.C. Anemia in People on Second Line Antiretroviral Treatment in Lilongwe, Malawi: A Cross-Sectional Study. BMC Infect. Dis. 2018, 18, 39. [Google Scholar] [CrossRef] [PubMed]
- Shevyrev, D.; Tereshchenko, V.; Berezina, T.N.; Rybtsov, S. Hematopoietic Stem Cells and the Immune System in Development and Aging. Int. J. Mol. Sci. 2023, 24, 5862. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Su, B.; Zhang, X.; Liu, Y.; Wu, H.; Zhang, T. Incomplete Immune Reconstitution in HIV/AIDS Patients on Antiretroviral Therapy: Challenges of Immunological Non-responders. J. Leukoc. Biol. 2020, 107, 597–612. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhao, J.; Cheng, L.; Jiang, Q.; Kan, S.; Qin, E.; Tu, B.; Zhang, X.; Zhang, L.; Su, L.; et al. HIV-1 Infection Depletes Human CD34+CD38- Hematopoietic Progenitor Cells via pDC-Dependent Mechanisms. PLOS Pathog. 2017, 13, e1006505. [Google Scholar] [CrossRef] [PubMed]
- Prost, S.; Dantec, M.L.; Augé, S.; Grand, R.L.; Derdouch, S.; Auregan, G.; Déglon, N.; Relouzat, F.; Aubertin, A.-M.; Maillere, B.; et al. Human and Simian Immunodeficiency Viruses Deregulate Early Hematopoiesis through a Nef/PPARγ/STAT5 Signaling Pathway in Macaques. J. Clin. Investig. 2008, 118, 1765–1775. [Google Scholar] [CrossRef] [PubMed]
- Akkina, R. New Insights into HIV Impact on Hematopoiesis. Blood 2013, 122, 2144–2146. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.C.; McNamara, L.A.; Onafuwa-Nuga, A.; Shackleton, M.; Riddell, J.; Bixby, D.; Savona, M.R.; Morrison, S.J.; Collins, K.L. HIV-1 Utilizes the CXCR4 Chemokine Receptor to Infect Multipotent Hematopoietic Stem and Progenitor Cells. Cell Host Microbe 2011, 9, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Renelt, S.; Schult-Dietrich, P.; Baldauf, H.-M.; Stein, S.; Kann, G.; Bickel, M.; Kielland-Kaisen, U.; Bonig, H.; Marschalek, R.; Rieger, M.A.; et al. HIV-1 Infection of Long-Lived Hematopoietic Precursors In Vitro and In Vivo. Cells 2022, 11, 2968. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, R.D.; Beckerman, K.P.; Schall, T.J.; McCune, J.M. CXCR4 and CCR5 Expression Delineates Targets for HIV-1 Disruption of T Cell Differentiation1. J. Immunol. 1998, 161, 3702–3710. [Google Scholar] [CrossRef] [PubMed]
- Maldarelli, F.; Wu, X.; Su, L.; Simonetti, F.R.; Shao, W.; Hill, S.; Spindler, J.; Ferris, A.L.; Mellors, J.W.; Kearney, M.F.; et al. Specific HIV Integration Sites Are Linked to Clonal Expansion and Persistence of Infected Cells. Science 2014, 345, 179. [Google Scholar] [CrossRef] [PubMed]
- Zaikos, T.D.; Terry, V.H.; Sebastian Kettinger, N.T.; Lubow, J.; Painter, M.M.; Virgilio, M.C.; Neevel, A.; Taschuk, F.; Onafuwa-Nuga, A.; McNamara, L.A.; et al. Hematopoietic Stem and Progenitor Cells Are a Distinct HIV Reservoir That Contributes to Persistent Viremia in Suppressed Patients. Cell Rep. 2018, 25, 3759–3773.e9. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, J.; Washington, K.; Uchida, N.; Phang, O.; Kang, E.M.; Hsieh, M.M.; Tisdale, J.F. Long-Term Vector Integration Site Analysis Following Retroviral Mediated Gene Transfer to Hematopoietic Stem Cells for the Treatment of HIV Infection. PLoS ONE 2009, 4, e4211. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, I.; Hashemi, F.B. Strategies to Eradicate HIV from Infected Patients: Elimination of Latent Provirus Reservoirs. Cell. Mol. Life Sci. 2019, 76, 3583–3600. [Google Scholar] [CrossRef] [PubMed]
- McNamara, L.A.; Ganesh, J.A.; Collins, K.L. Latent HIV-1 Infection Occurs in Multiple Subsets of Hematopoietic Progenitor Cells and Is Reversed by NF-κB Activation. J. Virol. 2012, 86, 9337–9350. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Wüthrich, M.; Klein, B.; Suresh, M. Indirect Regulation of CD4 T-Cell Responses by Tumor Necrosis Factor Receptors in an Acute Viral Infection. J. Virol. 2007, 81, 6502–6512. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Mayne, M.; Power, C.; Nath, A. The Tat Protein of HIV-1 Induces Tumor Necrosis Factor-α Production: Implications for Hiv-1-Associated Neurological Diseases. J. Biol. Chem. 1997, 272, 22385–22388. [Google Scholar] [CrossRef] [PubMed]
- Ensoli, B.; Buonaguro, L.; Barillari, G.; Fiorelli, V.; Gendelman, R.; Morgan, R.A.; Wingfield, P.; Gallo, R.C. Release, Uptake, and Effects of Extracellular Human Immunodeficiency Virus Type 1 Tat Protein on Cell Growth and Viral Transactivation. J. Virol. 1993, 67, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Tardieu, M.; Héry, C.; Peudenier, S.; Boespflug, O.; Montagnier, L. Human Immunodeficiency Virus Type 1–Infected Monocytic Cells Can Destroy Human Neural Cells after Cell-to-Cell Adhesion. Ann. Neurol. 1992, 32, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Chan, F.K.-M. Three Is Better Than One: Pre-Ligand Receptor Assembly in the Regulation of TNF Receptor Signaling. Cytokine 2007, 37, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Wajant, H.; Scheurich, P. TNFR1-Induced Activation of the Classical NF-κB Pathway. FEBS J. 2011, 278, 862–876. [Google Scholar] [CrossRef]
- Li, Z.-W.; Chu, W.; Hu, Y.; Delhase, M.; Deerinck, T.; Ellisman, M.; Johnson, R.; Karin, M. The IKKβ Subunit of IκB Kinase (IKK) Is Essential for Nuclear Factor κB Activation and Prevention of Apoptosis. J. Exp. Med. 1999, 189, 1839. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. Shared Principles in NF-kappaB Signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef] [PubMed]
- Scheidereit, C. IkappaB Kinase Complexes: Gateways to NF-kappaB Activation and Transcription. Oncogene 2006, 25, 6685–6705. [Google Scholar] [CrossRef] [PubMed]
- Osborn, L.; Kunkel, S.; Nabel, G.J. Tumor Necrosis Factor Alpha and Interleukin 1 Stimulate the Human Immunodeficiency Virus Enhancer by Activation of the Nuclear Factor Kappa B. Proc. Natl. Acad. Sci. USA 1989, 86, 2336–2340. [Google Scholar] [CrossRef] [PubMed]
- Folks, T.M.; Clouse, K.A.; Justement, J.; Rabson, A.; Duh, E.; Kehrl, J.H.; Fauci, A.S. Tumor Necrosis Factor Alpha Induces Expression of Human Immunodeficiency Virus in a Chronically Infected T-Cell Clone. Proc. Natl. Acad. Sci. USA 1989, 86, 2365–2368. [Google Scholar] [CrossRef] [PubMed]
- Gogoleva, V.S.; Atretkhany, K.-S.N.; Dygay, A.P.; Yurakova, T.R.; Drutskaya, M.S.; Nedospasov, S.A. Current Perspectives on the Role of TNF in Hematopoiesis Using Mice With Humanization of TNF/LT System. Front. Immunol. 2021, 12, 661900. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Passegué, E. TNFα Coordinates Hematopoietic Stem Cell Survival and Myeloid Regeneration. Cell Stem Cell 2019, 25, 357–372.e7. [Google Scholar] [CrossRef] [PubMed]
- Rundberg Nilsson, A.; Hidalgo, I.; Bryder, D.; Pronk, C.J. Temporal Dynamics of TNF-Mediated Changes in Hematopoietic Stem Cell Function and Recovery. iScience 2023, 26, 106341. [Google Scholar] [CrossRef] [PubMed]
- Mussbacher, M.; Salzmann, M.; Brostjan, C.; Hoesel, B.; Schoergenhofer, C.; Datler, H.; Hohensinner, P.; Basílio, J.; Petzelbauer, P.; Assinger, A.; et al. Cell Type-Specific Roles of NF-κB Linking Inflammation and Thrombosis. Front. Immunol. 2019, 10, 401678. [Google Scholar] [CrossRef]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef] [PubMed]
- Fiume, G.; Vecchio, E.; De Laurentiis, A.; Trimboli, F.; Palmieri, C.; Pisano, A.; Falcone, C.; Pontoriero, M.; Rossi, A.; Scialdone, A.; et al. Human Immunodeficiency Virus-1 Tat Activates NF-κB via Physical Interaction with IκB-α and P65. Nucleic Acids Res. 2012, 40, 3548–3562. [Google Scholar] [CrossRef] [PubMed]
- Min, A.K.; Fortune, T.; Rodriguez, N.; Hedge, E.; Swartz, T.H. Inflammasomes as Mediators of Inflammation in HIV-1 Infection. Transl. Res. 2023, 252, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Iannello, A.; Samarani, S.; Morisset, R.; Toma, E.; Grosley, M.; Ahmad, A. Contribution of Platelet Activation to Plasma IL-18 Concentrations in HIV-Infected AIDS Patients. AIDS 2006, 20, 1907–1909. [Google Scholar] [CrossRef] [PubMed]
- Gibellini, D.; Re, M.C.; Vitone, F.; Rizzo, N.; Maldini, C.; La Placa, M.; Zauli, G. Selective Up-Regulation of Functional CXCR4 Expression in Erythroid Cells by HIV-1 Tat Protein. Clin. Exp. Immunol. 2003, 131, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, D.; Uehira, T.; Yonemoto, H.; Bando, H.; Ogawa, Y.; Yajima, K.; Taniguchi, T.; Kasai, D.; Nishida, Y.; Shirasaka, T. Sustained High Levels of Serum Interferon-γ during HIV-1 Infection: A Specific Trend Different from Other Cytokines. Viral Immunol. 2010, 23, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Durandt, C.; Potgieter, J.; Mellet, J.; Herd, C.; Khoosal, R.; Nel, J.G.; Rossouw, T.; Pepper, M.S. HIV and Haematopoiesis. South Afr. Med. J. 2019, 109, 40. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, S.G.; Levin, B.R.; Bristol, G.; Rezek, V.; Kim, S.; Aguilera-Sandoval, C.; Balamurugan, A.; Yang, O.O.; Zack, J.A. In Vivo Suppression of HIV by Antigen Specific T Cells Derived from Engineered Hematopoietic Stem Cells. PLOS Pathog. 2012, 8, e1002649. [Google Scholar] [CrossRef] [PubMed]
- Redig, A.J.; Berliner, N. Pathogenesis and Clinical Implications of HIV-Related Anemia in 2013. Hematol. 2013 Am. Soc. Hematol. Educ. Program Book 2013, 2013, 377–381. [Google Scholar] [CrossRef] [PubMed]
- McNamara, L.A.; Collins, K.L. Hematopoietic Stem/Precursor Cells as HIV Reservoirs. Curr. Opin. HIV AIDS 2011, 6, 43–48. [Google Scholar] [CrossRef]
- Zou, W.; Xing, J.; Wang, F.; Chen, X.; Liu, Q.; Wang, J.; Zou, S.; Chen, L.; Fu, X.; Zhou, Z.; et al. HIV-1LAI Nef Blocks the Development of Hematopoietic Stem/Progenitor Cells into T Lymphoid Cells. AIDS 2021, 35, 851. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, V.; Tartaglia, E.; Refolo, G.; Sacchi, A.; Grassi, G.; Antinori, A.; Fimia, G.M.; Agrati, C. Per2 Upregulation in Circulating Hematopoietic Progenitor Cells During Chronic HIV Infection. Front. Cell. Infect. Microbiol. 2020, 10, 362. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Goldberg, M.A.; Scadden, D.T. HIV-1 Suppresses Erythropoietin Production in Vitro. Exp. Hematol. 1993, 21, 683–688. [Google Scholar] [PubMed]
- Morceau, F.; Dicato, M.; Diederich, M. Pro-Inflammatory Cytokine-Mediated Anemia: Regarding Molecular Mechanisms of Erythropoiesis. Mediat. Inflamm. 2010, 2009, e405016. [Google Scholar] [CrossRef]
- Jelkmann, W. Physiology and Pharmacology of Erythropoietin. Transfus. Med. Hemotherapy 2013, 40, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Onai, N.; Yoshihara, H.; Arai, F.; Suda, T.; Ohteki, T. Interferon Regulatory Factor-2 Protects Quiescent Hematopoietic Stem Cells from Type I Interferon–Dependent Exhaustion. Nat. Med. 2009, 15, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ling, F.; Wang, H.-C.; Sun, X.-H. Chronic TLR Signaling Impairs the Long-Term Repopulating Potential of Hematopoietic Stem Cells of Wild Type but Not Id1 Deficient Mice. PLoS ONE 2013, 8, e55552. [Google Scholar] [CrossRef]
- Canny, S.P.; Orozco, S.L.; Thulin, N.K.; Hamerman, J.A. Immune Mechanisms in Inflammatory Anemia. Annu. Rev. Immunol. 2023, 41, 405–429. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Anemia of Inflammation. N. Engl. J. Med. 2019, 381, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Akchurin, O.; Patino, E.; Dalal, V.; Meza, K.; Bhatia, D.; Brovender, S.; Zhu, Y.-S.; Cunningham-Rundles, S.; Perelstein, E.; Kumar, J.; et al. Interleukin-6 Contributes to the Development of Anemia in Juvenile CKD. Kidney Int. Rep. 2019, 4, 470. [Google Scholar] [CrossRef] [PubMed]
- Agus Somia, I.K.; Merati, T.P.; Bakta, I.M.; Putra Manuaba, I.B.; Yasa, W.P.S.; Sukrama, I.D.M.; Suryana, K.; Wisaksana, R. High Levels of Serum IL-6 and Serum Hepcidin and Low CD4 Cell Count Were Risk Factors of Anemia of Chronic Disease in HIV Patients on the Combination of Antiretroviral Therapy. HIV AIDS 2019, 11, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Ellaurie, M.; Rubinstein, A. Elevated Tumor Necrosis Factor-Alpha in Association with Severe Anemia in Human Immunodeficiency Virus Infection and Mycobacterium Avium Intracellulare Infection. Pediatr. Hematol. Oncol. 1995, 12, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Araújo-Pereira, M.; Barreto-Duarte, B.; Arriaga, M.B.; Musselwhite, L.W.; Vinhaes, C.L.; Belaunzaran-Zamudio, P.F.; Rupert, A.; Montaner, L.J.; Lederman, M.M.; Sereti, I.; et al. Relationship Between Anemia and Systemic Inflammation in People Living With HIV and Tuberculosis: A Sub-Analysis of the CADIRIS Clinical Trial. Front. Immunol. 2022, 13, 916216. [Google Scholar] [CrossRef] [PubMed]
- Vallurupalli, M.; MacFadyen, J.G.; Glynn, R.J.; Thuren, T.; Libby, P.; Berliner, N.; Ridker, P.M. Effects of Interleukin-1β Inhibition on Incident Anemia. Ann. Intern. Med. 2020, 172, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Lipshultz, H.M.; Hileman, C.O.; Ahuja, S.; Funderburg, N.T.; McComsey, G.A. Anemia Is Associated with Monocyte Activation in HIV-Infected Adults on Antiretroviral Therapy. Antivir. Ther. 2015, 20, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Quiros-Roldan, E.; Castelli, F.; Lanza, P.; Pezzoli, C.; Vezzoli, M.; Biasiotto, G.; Zanella, I. The Impact of Antiretroviral Therapy on Iron Homeostasis and Inflammation Markers in HIV-Infected Patients with Mild Anemia. J. Transl. Med. 2017, 15, 256. [Google Scholar] [CrossRef] [PubMed]
- Suresh, S.; Rajvanshi, P.K.; Noguchi, C.T. The Many Facets of Erythropoietin Physiologic and Metabolic Response. Front. Physiol. 2020, 10, 497186. [Google Scholar] [CrossRef] [PubMed]
- Araújo-Pereira, M.; Krishnan, S.; Salgame, P.; Manabe, Y.C.; Hosseinipour, M.C.; Bisson, G.; Severe, D.P.; Rouzier, V.; Leong, S.; Mave, V.; et al. Effect of the Relationship between Anaemia and Systemic Inflammation on the Risk of Incident Tuberculosis and Death in People with Advanced HIV: A Sub-Analysis of the REMEMBER Trial. eClinicalMedicine 2023, 60, 102030. [Google Scholar] [CrossRef] [PubMed]
- Demitto, F.O.; Araújo-Pereira, M.; Schmaltz, C.A.; Sant’Anna, F.M.; Arriaga, M.B.; Andrade, B.B.; Rolla, V.C. Impact of Persistent Anemia on Systemic Inflammation and Tuberculosis Outcomes in Persons Living With HIV. Front. Immunol. 2020, 11, 588405. [Google Scholar] [CrossRef] [PubMed]
- Vinhaes, C.L.; Araujo-Pereira, M.; Tibúrcio, R.; Cubillos-Angulo, J.M.; Demitto, F.O.; Akrami, K.M.; Andrade, B.B. Systemic Inflammation Associated with Immune Reconstitution Inflammatory Syndrome in Persons Living with HIV. Life 2021, 11, 65. [Google Scholar] [CrossRef] [PubMed]
- Boulware, D.R.; Hullsiek, K.H.; Puronen, C.E.; Rupert, A.; Baker, J.V.; French, M.A.; Bohjanen, P.R.; Novak, R.M.; Neaton, J.D.; Sereti, I. Higher Levels of CRP, D-Dimer, IL-6, and Hyaluronic Acid Before Initiation of Antiretroviral Therapy (ART) Are Associated With Increased Risk of AIDS or Death. J. Infect. Dis. 2011, 203, 1637–1646. [Google Scholar] [CrossRef] [PubMed]
- Shivakoti, R.; Yang, W.-T.; Gupte, N.; Berendes, S.; Rosa, A.L.; Cardoso, S.W.; Mwelase, N.; Kanyama, C.; Pillay, S.; Samaneka, W.; et al. Concurrent Anemia and Elevated C-Reactive Protein Predicts HIV Clinical Treatment Failure, Including Tuberculosis, After Antiretroviral Therapy Initiation. Clin. Infect. Dis. 2015, 61, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Mollel, E.W.; Todd, J.; Mahande, M.J.; Msuya, S.E. Effect of Tuberculosis Infection on Mortality of HIV-Infected Patients in Northern Tanzania. Trop. Med. Health 2020, 48, 26. [Google Scholar] [CrossRef] [PubMed]
- Giganti, M.J.; Limbada, M.; Mwango, A.; Moyo, C.; Mulenga, L.B.; Guffey, M.B.; Mulenga, P.L.; Bolton-Moore, C.; Stringer, J.S.A.; Chi, B.H. Six-Month Hemoglobin Concentration and Its Association with Subsequent Mortality among Adults on Antiretroviral Therapy in Lusaka, Zambia. J. Acquir. Immune Defic. Syndr. 2012, 61, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Kallianpur, A.R.; Wang, Q.; Jia, P.; Hulgan, T.; Zhao, Z.; Letendre, S.L.; Ellis, R.J.; Heaton, R.K.; Franklin, D.R.; Barnholtz-Sloan, J.; et al. Anemia and Red Blood Cell Indices Predict HIV-Associated Neurocognitive Impairment in the Highly Active Antiretroviral Therapy Era. J. Infect. Dis. 2016, 213, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.G.; Palella, F.J.; Li, X.; Estrella, M.M.; Kingsley, L.A.; Witt, M.D.; Jacobson, L.P. The Impact of Impaired Kidney Function and HIV Infection on the Risk of Anemia. AIDS Res. Hum. Retroviruses 2012, 28, 1666–1671. [Google Scholar] [CrossRef] [PubMed]
- Belay, A.S.; Manaye, G.A.; Kebede, K.M.; Abateneh, D.D.; Debebe, S. Chronic Kidney Disease and Its Predictors among Highly Active Antiretroviral Therapy Naïve and Experienced HIV-Infected Individuals at the Selected Hospitals, Southwest Ethiopia: A Comparative Cross-Sectional Study. BMJ Public Health 2023, 1. [Google Scholar] [CrossRef]
- Boswell, M.T.; Rossouw, T.M. Approach to Acute Kidney Injury in HIV-Infected Patients in South Africa. South. Afr. J. HIV Med. 2017, 18, 714. [Google Scholar] [CrossRef] [PubMed]
- Tsuro, U.; Oladimeji, K.E.; Pulido-Estrada, G.-A.; Apalata, T.R. Risk Factors Attributable to Hypertension among HIV-Infected Patients on Antiretroviral Therapy in Selected Rural Districts of the Eastern Cape Province, South Africa. Int. J. Environ. Res. Public. Health 2022, 19, 11196. [Google Scholar] [CrossRef] [PubMed]
- Nyende, L.; Kalyesubula, R.; Sekasanvu, E.; Byakika-Kibwika, P. Prevalence of Renal Dysfunction among HIV Infected Patients Receiving Tenofovir at Mulago: A Cross-Sectional Study. BMC Nephrol. 2020, 21, 232. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, J.; Floege, J.; Fliser, D.; Böhm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; He, Y.; Zhang, J.; Hong, D.; Li, G. Erythropoietin and Iron for Anemia in HIV-Infected Patients Undergoing Maintenance Hemodialysis in China: A Cross-Sectional Study. BMC Nephrol. 2022, 23, 60. [Google Scholar] [CrossRef] [PubMed]
- Echefu, G.; Stowe, I.; Burka, S.; Basu-Ray, I.; Kumbala, D. Pathophysiological Concepts and Screening of Cardiovascular Disease in Dialysis Patients. Front. Nephrol. 2023, 3, 1198560. [Google Scholar] [CrossRef] [PubMed]
- Emem, C.P.; Arogundade, F.; Sanusi, A.; Adelusola, K.; Wokoma, F.; Akinsola, A. Renal Disease in HIV-Seropositive Patients in Nigeria: An Assessment of Prevalence, Clinical Features and Risk Factors. Nephrol. Dial. Transplant. 2008, 23, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Kavishe, B.B.; Kweka, B.V.; Nitsch, D.; PrayGod, G.; Jeremiah, K.; Faurholt-Jepsen, D.; Filteau, S.; Olsen, M.F.; Kitilya, B.W.; Krogh-Madsen, R.; et al. Risk Factors for Impaired Renal Function in HIV-Infected and HIV-Uninfected Adults: Cross-Sectional Study in North-Western Tanzania. BMC Nephrol. 2021, 22, 355. [Google Scholar] [CrossRef] [PubMed]
- Begum, S.; Latunde-Dada, G.O. Anemia of Inflammation with An Emphasis on Chronic Kidney Disease. Nutrients 2019, 11, 2424. [Google Scholar] [CrossRef] [PubMed]
- Kadatane, S.P.; Satariano, M.; Massey, M.; Mongan, K.; Raina, R. The Role of Inflammation in CKD. Cells 2023, 12, 1581. [Google Scholar] [CrossRef] [PubMed]
- Meremo, A.J.; Mwashambwa, M.Y.; Masalu, M.B.; Kapinga, J.; Tagalile, R.; Ngilangwa, D.P.; Sabi, I. Prevalence and Predictors of Anaemia among Patients Presenting with Kidney Diseases at the University of Dodoma Hospital in Central Tanzania. Tanzan. J. Health Res. 2017, 19. [Google Scholar] [CrossRef]
- Imig, J.D.; Ryan, M.J. Immune and Inflammatory Role in Renal Disease. Compr. Physiol. 2013, 3, 957–976. [Google Scholar] [CrossRef] [PubMed]
- Stenvinkel, P.; Chertow, G.M.; Devarajan, P.; Levin, A.; Andreoli, S.P.; Bangalore, S.; Warady, B.A. Chronic Inflammation in Chronic Kidney Disease Progression: Role of Nrf2. Kidney Int. Rep. 2021, 6, 1775–1787. [Google Scholar] [CrossRef] [PubMed]
- Moranne, O.; Froissart, M.; Rossert, J.; Gauci, C.; Boffa, J.-J.; Haymann, J.P.; M’rad, M.B.; Jacquot, C.; Houillier, P.; Stengel, B.; et al. Timing of Onset of CKD-Related Metabolic Complications. J. Am. Soc. Nephrol. 2009, 20, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Grabovac, I.; Veronese, N.; Stefanac, S.; Haider, S.; Jackson, S.E.; Koyanagi, A.; Meilinger, M.; Stubbs, B.; Firth, J.; Soysal, P.; et al. Human Immunodeficiency Virus Infection and Diverse Physical Health Outcomes: An Umbrella Review of Meta-Analyses of Observational Studies. Clin. Infect. Dis. 2020, 70, 1809–1815. [Google Scholar] [CrossRef] [PubMed]
- Nou, E.; Lo, J.; Grinspoon, S.K. Inflammation, Immune Activation, and Cardiovascular Disease in HIV. AIDS 2016, 30, 1495–1509. [Google Scholar] [CrossRef] [PubMed]
- Perkins, M.V.; Joseph, S.B.; Dittmer, D.P.; Mackman, N. Cardiovascular Disease and Thrombosis in HIV Infection. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, C.; Tousoulis, D. Inflammatory Mechanisms Contributing to Endothelial Dysfunction. Biomedicines 2021, 9, 781. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.H.; Kime, N.; Korcarz, C.E.; Ribaudo, H.; Currier, J.S.; Delaney, J.C. Effects of HIV Infection on Arterial Endothelial Function. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Harding, B.N.; Whitney, B.M.; Nance, R.M.; Crane, H.M.; Burkholder, G.; Moore, R.D.; Mathews, W.C.; Eron, J.J.; Hunt, P.W.; Volberding, P.; et al. Antiretroviral Drug Class and Anaemia Risk in the Current Treatment Era among People Living with HIV in the USA: A Clinical Cohort Study. BMJ Open 2020, 10, e031487. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Li, R.; Zhang, J.; Zhang, L.; Yang, M.; Zhang, Q.; Li, H. Association of Myocardial Iron Deficiency Based on T2* CMR with the Risk of Mild Left Ventricular Dysfunction in HIV-1-Infected Patients. Front. Cardiovasc. Med. 2023, 10, 1132893. [Google Scholar] [CrossRef] [PubMed]
- Caruso, C.; Fay, M.E.; Cheng, X.; Liu, A.Y.; Park, S.I.; Sulchek, T.A.; Graham, M.D.; Lam, W.A. Pathologic Mechanobiological Interactions between Red Blood Cells and Endothelial Cells Directly Induce Vasculopathy in Iron Deficiency Anemia. iScience 2022, 25, 104606. [Google Scholar] [CrossRef] [PubMed]
- Lanser, L.; Fuchs, D.; Scharnagl, H.; Grammer, T.; Kleber, M.E.; März, W.; Weiss, G.; Kurz, K. Anemia of Chronic Disease in Patients With Cardiovascular Disease. Front. Cardiovasc. Med. 2021, 8, 666638. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.R.; Rachel, G.; Parthasarathy, D. HIV Proteins and Endothelial Dysfunction: Implications in Cardiovascular Disease. Front. Cardiovasc. Med. 2018, 5, 185. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Chai, H.; Lin, P.H.; Yao, Q.; Chen, C. Current Update on HIV-Associated Vascular Disease and Endothelial Dysfunction. World J. Surg. 2007, 31, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.V.; Valuev-Elliston, V.T.; Ivanova, O.N.; Kochetkov, S.N.; Starodubova, E.S.; Bartosch, B.; Isaguliants, M.G. Oxidative Stress during HIV Infection: Mechanisms and Consequences. Oxid. Med. Cell Longev. 2016, 2016, 8910396. [Google Scholar] [CrossRef] [PubMed]
- Bozzette, S.A.; Ake, C.F.; Tam, H.K.; Chang, S.W.; Louis, T.A. Cardiovascular and Cerebrovascular Events in Patients Treated for Human Immunodeficiency Virus Infection. N. Engl. J. Med. 2003, 348, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Currier, J.S.; Lundgren, J.D.; Carr, A.; Klein, D.; Sabin, C.A.; Sax, P.E.; Schouten, J.T.; Smieja, M.; Working Group 2. Epidemiological Evidence for Cardiovascular Disease in HIV-Infected Patients and Relationship to Highly Active Antiretroviral Therapy. Circulation 2008, 118, e29–e35. [Google Scholar] [CrossRef] [PubMed]
- Suja, S.; Saravanan, T.; Karthikeyan, S. Profile of Hematological Abnormalities and Its Correlation with Absolute CD4 Count and Human Immunodeficiency Virus Viral Load in Human Immunodeficiency Virus-Infected Patients in a Tertiary Care Hospital. Indian J. Sex. Transm. Dis. AIDS 2020, 41, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Lumsden, R.H.; Bloomfield, G.S. The Causes of HIV-Associated Cardiomyopathy: A Tale of Two Worlds. Biomed. Res. Int. 2016, 2016, 8196560. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Dey, A.K.; Sharma, G.; Bhoite, R.; Burkholder, G.; Fedson, S.; Jneid, H. Etiology and Pathophysiology of Heart Failure in People with HIV. Heart Fail. Rev. 2021, 26, 497–505. [Google Scholar] [CrossRef]
- Janaszak-Jasiecka, A.; Siekierzycka, A.; Płoska, A.; Dobrucki, I.T.; Kalinowski, L. Endothelial Dysfunction Driven by Hypoxia-The Influence of Oxygen Deficiency on NO Bioavailability. Biomolecules 2021, 11, 982. [Google Scholar] [CrossRef] [PubMed]
- Medina-Leyte, D.J.; Zepeda-García, O.; Domínguez-Pérez, M.; González-Garrido, A.; Villarreal-Molina, T.; Jacobo-Albavera, L. Endothelial Dysfunction, Inflammation and Coronary Artery Disease: Potential Biomarkers and Promising Therapeutical Approaches. Int. J. Mol. Sci. 2021, 22, 3850. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.A.; Sarnak, M.J. Anemia as a Risk Factor for Cardiovascular Disease: Management of Comorbidities in Kidney Disease in the 21st Century: Anemia and Bone Disease. Kidney Int. 2003, 64, S32–S39. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, E.A.; von Haehling, S.; Anker, S.D.; Macdougall, I.C.; Ponikowski, P. Iron Deficiency and Heart Failure: Diagnostic Dilemmas and Therapeutic Perspectives. Eur. Heart J. 2013, 34, 816–829. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Tsujino, T.; Matsumoto, M.; Sakoda, T.; Ohyanagi, M.; Masuyama, T. Adaptive Response of the Heart to Long-Term Anemia Induced by Iron Deficiency. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H585–H593. [Google Scholar] [CrossRef] [PubMed]
- Chennupati, R.; Solga, I.; Wischmann, P.; Dahlmann, P.; Celik, F.G.; Pacht, D.; Şahin, A.; Yogathasan, V.; Hosen, M.R.; Gerdes, N.; et al. Chronic Anemia Is Associated with Systemic Endothelial Dysfunction. Front. Cardiovasc. Med. 2023, 10, 1099069. [Google Scholar] [CrossRef] [PubMed]
- Wischmann, P.; Kuhn, V.; Suvorava, T.; Muessig, J.M.; Fischer, J.W.; Isakson, B.E.; Haberkorn, S.M.; Flögel, U.; Schrader, J.; Jung, C.; et al. Anaemia Is Associated with Severe RBC Dysfunction and a Reduced Circulating NO Pool: Vascular and Cardiac eNOS Are Crucial for the Adaptation to Anaemia. Basic. Res. Cardiol. 2020, 115, 43. [Google Scholar] [CrossRef] [PubMed]
- Andrabi, S.M.; Sharma, N.S.; Karan, A.; Shahriar, S.M.S.; Cordon, B.; Ma, B.; Xie, J. Nitric Oxide: Physiological Functions, Delivery, and Biomedical Applications. Adv. Sci. 2023, 10, e2303259. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of Nitric Oxide in Inflammatory Diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Iwata, M.; Inoue, T.; Asai, Y.; Hori, K.; Fujiwara, M.; Matsuo, S.; Tsuchida, W.; Suzuki, S. The Protective Role of Localized Nitric Oxide Production during Inflammation May Be Mediated by the Heme Oxygenase-1/Carbon Monoxide Pathway. Biochem. Biophys. Rep. 2020, 23, 100790. [Google Scholar] [CrossRef]
- Förstermann, U.; Münzel, T. Endothelial Nitric Oxide Synthase in Vascular Disease. Circulation 2006, 113, 1708–1714. [Google Scholar] [CrossRef] [PubMed]
- Landmesser, U.; Hornig, B.; Drexler, H. Endothelial Function. Circulation 2004, 109, II-27. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.K.; Donnelly, E.; Islam, K.N. Circulating Hydrogen Sulfide (H2S) and Nitric Oxide (NO) Levels Are Significantly Reduced in HIV Patients Concomitant with Increased Oxidative Stress Biomarkers. J. Clin. Med. 2021, 10, 4460. [Google Scholar] [CrossRef] [PubMed]
- Paladugu, R.; Fu, W.; Conklin, B.S.; Lin, P.H.; Lumsden, A.B.; Yao, Q.; Chen, C. Hiv Tat Protein Causes Endothelial Dysfunction in Porcine Coronary Arteries. J. Vasc. Surg. 2003, 38, 549–555; discussion 555–556. [Google Scholar] [CrossRef] [PubMed]
- Nagababu, E.; Gulyani, S.; Earley, C.J.; Cutler, R.G.; Mattson, M.P.; Rifkind, J.M. Iron-Deficiency Anemia Enhances Red Blood Cell Oxidative Stress. Free Radic. Res. 2008, 42, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Dunaway, L. Endothelial Hemoglobin Alpha Mediates Iron Regulation of Endothelial Nitric Oxide. Blood 2023, 142, 502. [Google Scholar] [CrossRef]
- Darmada, P.D.; Suryana, K. The The Impact of Antiretroviral Therapy on Hemoglobin Levels of Hiv/Aids Patients At Merpati Clinic, Wangaya Hospital, Denpasar, Bali, Indonesia. Int. J. Pharm. Pharm. Sci. 2020, 12, 13–16. [Google Scholar] [CrossRef]
- Xie, B.; Huang, W.; Hu, Y.; Dou, Y.; Xie, L.; Zhang, Y.; Qin, S.; Lan, K.; Pang, X.; Qiu, H.; et al. Anemia and Opportunistic Infections in Hospitalized People Living with HIV: A Retrospective Study. BMC Infect. Dis. 2022, 22, 912. [Google Scholar] [CrossRef] [PubMed]
- Gargallo-Puyuelo, C.J.; Alfambra, E.; García-Erce, J.A.; Gomollon, F. Iron Treatment May Be Difficult in Inflammatory Diseases: Inflammatory Bowel Disease as a Paradigm. Nutrients 2018, 10, 1959. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Brookes, M.J. Iron Therapy in Inflammatory Bowel Disease. Nutrients 2020, 12, 3478. [Google Scholar] [CrossRef] [PubMed]
- Obeagu, E.I.; Obeagu, G.U.; Ukibe, N.R.; Oyebadejo, S.A. Anemia, Iron, and HIV: Decoding the Interconnected Pathways: A Review. Medicine 2024, 103, e36937. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Donovan, K.; Seeley, C.; Dickson, E.A.; Palmer, A.J.R.; Doree, C.; Brunskill, S.; Reid, J.; Acheson, A.G.; Sugavanam, A.; et al. Risk of Infection Associated With Administration of Intravenous Iron: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2021, 4, e2133935. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.T.; Duggan, C.P.; Manji, K.; Seage, G.R.; Spiegelman, D.; Perumal, N.; Ulenga, N.; Fawzi, W.W. Iron Supplementation and Paediatric HIV Disease Progression: A Cohort Study among Children Receiving Routine HIV Care in Dar Es Salaam, Tanzania. Int. J. Epidemiol. 2022, 51, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Mhaskar, R.; Wao, H.; Miladinovic, B.; Kumar, A.; Djulbegovic, B. The Role of Iron in the Management of Chemotherapy-induced Anemia in Cancer Patients Receiving Erythropoiesis-stimulating Agents. Cochrane Database Syst. Rev. 2016, 2016, CD009624. [Google Scholar] [CrossRef] [PubMed]
- Sagar, P.; Angmo, S.; Sandhir, R.; Rishi, V.; Yadav, H.; Singhal, N.K. Effect of Hepcidin Antagonists on Anemia during Inflammatory Disorders. Pharmacol. Ther. 2021, 226, 107877. [Google Scholar] [CrossRef] [PubMed]
- Madu, A.J.; Ughasoro, M.D. Anaemia of Chronic Disease: An In-Depth Review. Med. Princ. Pract. 2016, 26, 1–9. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamvuma, K.; Hamooya, B.M.; Munsaka, S.; Masenga, S.K.; Kirabo, A. Mechanisms and Cardiorenal Complications of Chronic Anemia in People with HIV. Viruses 2024, 16, 542. https://doi.org/10.3390/v16040542
Kamvuma K, Hamooya BM, Munsaka S, Masenga SK, Kirabo A. Mechanisms and Cardiorenal Complications of Chronic Anemia in People with HIV. Viruses. 2024; 16(4):542. https://doi.org/10.3390/v16040542
Chicago/Turabian StyleKamvuma, Kingsley, Benson M. Hamooya, Sody Munsaka, Sepiso K. Masenga, and Annet Kirabo. 2024. "Mechanisms and Cardiorenal Complications of Chronic Anemia in People with HIV" Viruses 16, no. 4: 542. https://doi.org/10.3390/v16040542