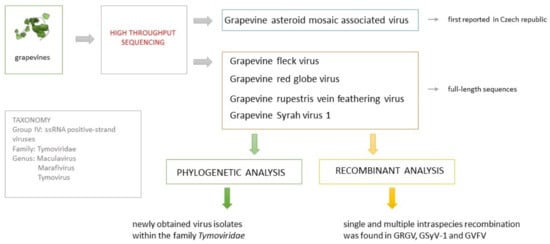
Multiple Infections with Viruses of the Family Tymoviridae in Czech Grapevines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and RT-PCR
2.2. High-Throughput Sequencing (HTS)
2.3. Bioinformatic Analysis
2.4. Recombination, Phylogenetic, and Sequence Demarcation Analyses
3. Results
3.1. Sequencing of RT-PCR Products Obtained with Generic Primers for the Family Tymoviridae
3.2. HTS Data Analysis and Identification of Viruses
3.3. Grapevine Fleck Virus (GFkV)
3.4. Grapevine Red Globe Virus (GRGV)
3.5. Grapevine Rupestris Vein Feathering Virus (GRVFV)
3.6. Grapevine Syrah Virus-1 (GSyV-1)
3.7. Grapevine Asteroid Mosaic-Associated Virus (GAMaV)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fuchs, M. Grapevine Viruses: A Multitude of Diverse Species with Simple but Overall Poorly Adopted Management Solutions in the Vineyard. J. Plant Pathol. 2020, 102, 643–653. [Google Scholar] [CrossRef]
- Miljanić, V.; Jakše, J.; Kunej, U.; Rusjan, D.; Škvarč, A.; Štajner, N. Virome Status of Preclonal Candidates of Grapevine Varieties (Vitis vinifera L.) from the Slovenian Wine-Growing Region Primorska as Determined by High-Throughput Sequencing. Front. Microbiol. 2022, 13, 830866. [Google Scholar] [CrossRef]
- Shvets, D.; Porotikova, E.; Sandomirsky, K.; Vinogradova, S. Virome of Grapevine Germplasm from the Anapa Ampelographic Collection (Russia). Viruses 2022, 14, 1314. [Google Scholar] [CrossRef]
- Vinogradova, S.; Porotikova, E.; Navrotskaya, E.; Galbacs, Z.N.; Massart, S.; Varallyay, E. The First Virome of a Russian Vineyard. Plants 2023, 12, 3292. [Google Scholar] [CrossRef]
- Czotter, N.; Molnar, J.; Szabó, E.; Demian, E.; Kontra, L.; Baksa, I.; Szittya, G.; Kocsis, L.; Deak, T.; Bisztray, G.; et al. NGS of Virus-Derived Small RNAs as a Diagnostic Method Used to Determine Viromes of Hungarian Vineyards. Front. Microbiol. 2018, 9, 122. [Google Scholar] [CrossRef]
- Fajardo, T.V.M.; Silva, F.N.; Eiras, M.; Nickel, O. High-Throughput Sequencing Applied for the Identification of Viruses Infecting Grapevines in Brazil and Genetic Variability Analysis. Trop. Plant Pathol. 2017, 42, 250–260. [Google Scholar] [CrossRef]
- Eichmeier, A.; Komínková, M.; Komínek, P.; Baránek, M. Comprehensive Virus Detection Using next Generation Sequencing in Grapevine Vascular Tissues of Plants Obtained from the Wine Regions of Bohemia and Moravia (Czech Republic). PLoS ONE 2016, 11, e0167966. [Google Scholar] [CrossRef]
- Glasa, M.; Predajňa, L.; Komínek, P.; Nagyová, A.; Candresse, T.; Olmos, A. Molecular Characterization of Divergent Grapevine Pinot Gris Virus Isolates and Their Detection in Slovak and Czech Grapevines. Arch. Virol. 2014, 159, 2103–2107. [Google Scholar] [CrossRef]
- Komínek, P. Distribution of Grapevine Viruses in Vineyards of the Czech Republic. J. Plant Pathol. 2008, 90, 357–358. [Google Scholar]
- Walker, P.J.; Siddell, S.G.; Lefkowitz, E.J.; Mushegian, A.R.; Adriaenssens, E.M.; Alfenas-Zerbini, P.; Davison, A.J.; Dempsey, D.M.; Dutilh, B.E.; García, M.L.; et al. Changes to Virus Taxonomy and to the International Code of Virus Classification and Nomenclature Ratified by the International Committee on Taxonomy of Viruses (2021). Arch. Virol. 2021, 166, 2633–2648. [Google Scholar] [CrossRef]
- Zerbini, F.M.; Siddell, S.G.; Mushegian, A.R.; Walker, P.J.; Lefkowitz, E.J.; Adriaenssens, E.M.; Alfenas-Zerbini, P.; Dutilh, B.E.; García, M.L.; Junglen, S.; et al. Differentiating between Viruses and Virus Species by Writing Their Names Correctly. Arch. Virol. 2022, 167, 1231–1234. [Google Scholar] [CrossRef]
- Sabanadzovic, S.; Abou-ghanem, N.; Martelli, G.P. Grapevine Fleck and Similar Viruses. In Grapevine Viruses: Molecular Biology, Diagnostics and Management; Meng, B., Martelli, G.P., Fuchs, M., Golino, D.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 167–195. ISBN 9783319577067. [Google Scholar]
- Jo, Y.; Song, M.K.; Choi, H.; Park, J.S.; Lee, J.W.; Cho, W.K. First Report of Grapevine Fleck Virus and Grapevine Virus E in Grapevine in Korea. Plant Dis. 2017, 101, 1069. [Google Scholar] [CrossRef]
- Crnogorac, A.; Gašpar, M.; Davino, S.; Mandić, A.; Matić, S. First Report of Grapevine Fleck Virus in Vineyards of Bosnia and Herzegovina. J. Plant Pathol. 2020, 102, 1299. [Google Scholar] [CrossRef]
- Komínek, P.; Holleinová, V. Evaluation of Sanitary Status of Grapevines in the Czech Republic. Plant Soil Environ. 2003, 49, 63–66. [Google Scholar] [CrossRef]
- Shi, B.J.; Habili, N.; Symons, R.H. Nucleotide Sequence Variation in a Small Region of the Grapevine Fleck Virus Replicase Provides Evidence for Two Sequence Variants of the Virus. Ann. Appl. Biol. 2003, 142, 349–355. [Google Scholar] [CrossRef]
- Martelli, G.P.; Sabanadzovic, S.; Ghanem-Sabanadzovic, N.A.; Saldarelli, P. Maculavirus, a New Genus of Plant Viruses. Arch. Virol. 2002, 147, 1847–1853. [Google Scholar] [CrossRef]
- Sabanadzovic, S.; Abou-Ghanem, N.; Castellano, M.A.; Digiaro, M.; Martelli, G.P. Grapevine Fleck Virus-like Viruses in Vitis. Arch. Virol. 2000, 145, 553–565. [Google Scholar] [CrossRef]
- Ghanem-Sabanadzovic, N.A.; Sabanadzovic, S.; Martelli, G.P. Sequence Analysis of the 3′ End of Three Grapevine Fleck Virus-like Viruses from Grapevine. Virus Genes 2003, 27, 11–16. [Google Scholar] [CrossRef]
- El Beaino, T.; Sabanadzovic, S.; Digiaro, M.; Abou Ghanem-Sabanadzovic, N.; Rowhani, A.; Kyriakopoulou, P.E.; Martelli, G.P. Molecular Detection of Grapevine Fleck Virus-like Viruses. Vitis 2001, 40, 65–68. [Google Scholar]
- Beuve, M.; Candresse, T.; Tannières, M.; Lemaire, O. First Report of Grapevine Redglobe Virus (GRGV) in Grapevine in France. Plant Dis. 2015, 99, 422. [Google Scholar] [CrossRef] [PubMed]
- Cretazzo, E.; Padilla, C.V.; Velasco, L. First Report of Grapevine Red Globe Virus in Grapevine in Spain. Plant Dis. 2017, 101, 264–265. [Google Scholar] [CrossRef]
- Ruiz-García, A.B.; Zarghani, S.N.; Okic, A.; Olmos, A.; Wetzel, T. First Report of Grapevine Red Globe Virus in Grapevine in Germany. Plant Dis. 2018, 102, 1675. [Google Scholar] [CrossRef]
- Candresse, T.; Faure, C.; Marais, A. First Report of Grapevine Red Globe Virus (GRGV) and Grapevine Rupestris Vein Feathering Virus (GRVFV) Infecting Grapevine (Vitis vinifera) in Portugal. Plant Dis. 2022, 107, 974. [Google Scholar] [CrossRef]
- Dixon, M.; Fowkes, A.; Hogan, C.; Adams, I.; McGreig, S.; Pufal, H.; Ward, R.; Harju, V.; Skelton, A.; Fox, A. First Report of Grapevine Red Globe Virus in Grapevine in the United Kingdom. New Dis. Rep. 2022, 46, e12118. [Google Scholar] [CrossRef]
- Fan, X.D.; Dong, Y.F.; Zhang, Z.P.; Ren, F.; Hu, G.J.; Li, Z.N.; Zhou, J. First Report of Grapevine Red Globe Virus (GRGV) in Grapevines in China. Plant Dis. 2016, 100, 2340. [Google Scholar] [CrossRef]
- Nourinejhad Zarghani, S.; Khalili, M.; Dizadji, A.; Wetzel, T. First Report of Grapevine Red Globe Virus in Grapevine in Iran. J. Plant Pathol. 2021, 103, 661. [Google Scholar] [CrossRef]
- Yamamoto, T.; Sato, H.; Suzuki, T.; Miyazaki, A.; Kitazawa, Y.; Maejima, K.; Namba, S.; Yamaji, Y. Complete Genome Sequences of Grapevine Red Globe Virus in Japan. Microbiol. Resour. Announc. 2022, 11, 12–14. [Google Scholar] [CrossRef]
- Wu, Q.; Habili, N.; Tyerman, S.D.; Rinaldo, A.; Little, A.; Constable, F.E. First Detection of Five Previously Unreported Grapevine Viruses in Australia. Australas. Plant Dis. Notes 2023, 18, 27. [Google Scholar] [CrossRef]
- Massart, S.; Chiumenti, M.; De Jonghe, K.; Glover, R.; Haegeman, A.; Koloniuk, I.; Komínek, P.; Kreuze, J.; Kutnjak, D.; Lotos, L.; et al. Virus Detection by High-Throughput Sequencing of Small RNAs: Large-Scale Performance Testing of Sequence Analysis Strategies. Phytopathology 2019, 109, 488–497. [Google Scholar] [CrossRef]
- Wu, Q.; Kehoe, M.A.; Kinoti, W.M.; Wang, C.P.; Rinaldo, A.; Tyerman, S.; Habili, N.; Constable, F.E. First Report of Grapevine Rupestris Vein Feathering Virus in Grapevine in Australia. Plant Dis. 2021, 105, 515. [Google Scholar] [CrossRef]
- Al Rwahnih, M.; Daubert, S.; Golino, D.; Rowhani, A. Deep Sequencing Analysis of RNAs from a Grapevine Showing Syrah Decline Symptoms Reveals a Multiple Virus Infection That Includes a Novel Virus. Virology 2009, 387, 395–401. [Google Scholar] [CrossRef]
- Engel, E.A.; Rivera, P.A.; Valenzuela, P.D.T. First Report of Grapevine Syrah Virus-1 in Chilean Grapevines. Plant Dis. 2010, 94, 633. [Google Scholar] [CrossRef]
- Giampetruzzi, A.; Roumi, V.; Roberto, R.; Malossini, U.; Yoshikawa, N.; La Notte, P.; Terlizzi, F.; Credi, R.; Saldarelli, P. A New Grapevine Virus Discovered by Deep Sequencing of Virus- and Viroid-Derived Small RNAs in Cv Pinot Gris. Virus Res. 2012, 163, 262–268. [Google Scholar] [CrossRef]
- Czotter, N.; Szabo, E.; Molnar, J.; Kocisz, L.; Deák, T.; Bisztray, G.; Tusnády, G.; Burgyán, J.; Várallyay, É. First Description of Grapevine Syrah Virus-1 from Grapevine in Hungary. J. Plant Pathol. 2015, 97, S74. [Google Scholar] [CrossRef]
- Oosthuizen, K.; Coetzee, B.; Maree, H.J.; Burger, J.T. First Report of Grapevine Syrah Virus 1 in South African Grapevines. Plant Dis. 2016, 100, 1252. [Google Scholar] [CrossRef]
- Ahmed, I.; Fan, X.D.; Zhang, Z.P.; Ren, F.; Hu, G.J.; Li, Z.N.; Khaskheli, M.I.; Dong, Y.F. First Report of Grapevine Syrah Virus-1 in Grapevines in China. Plant Dis. 2018, 102, 466. [Google Scholar] [CrossRef]
- Vončina, D.; Al Rwahnih, M.; Rowhani, A.; Gouran, M.; Almeida, R.P.P. Viral Diversity in Autochthonous Croatian Grapevine Cultivars. Plant Dis. 2017, 101, 1230–1235. [Google Scholar] [CrossRef]
- Ruiz-García, A.B.; Sabaté, J.; Lloria, O.; Laviña, A.; Batlle, A.; Olmos, A. First Report of Grapevine Syrah Virus-1 in Grapevine in Spain. Plant Dis. 2017, 101, 1830. [Google Scholar] [CrossRef]
- Cho, I.S.; Yang, C.Y.; Kwon, S.J.; Yoon, J.Y.; Kim, D.H.; Choi, G.S.; Hammond, J.; Moon, J.S.; Lim, H.S. First Report of Grapevine Syrah Virus 1 Infecting Grapevines in Korea. Plant Dis. 2019, 103, 2970. [Google Scholar] [CrossRef]
- Navrotskaya, E.; Porotikova, E.; Yurchenko, E.; Galbacs, Z.N.; Varallyay, E.; Vinogradova, S. High-Throughput Sequencing of Small Rnas for Diagnostics of Grapevine Viruses and Viroids in Russia. Viruses 2021, 13, 2432. [Google Scholar] [CrossRef]
- Glasa, M.; Predajňa, L.; Šoltys, K.; Sabanadzovic, S.; Olmos, A. Detection and Molecular Characterisation of Grapevine Syrah Virus-1 Isolates from Central Europe. Virus Genes 2015, 51, 112–121. [Google Scholar] [CrossRef]
- Sabanadzovic, S.; Ghanem-Sabanadzovic, N.A.; Gorbalenya, A.E. Permutation of the Active Site of Putative RNA-Dependent RNA Polymerase in a Newly Identified Species of Plant Alpha-like Virus. Virology 2009, 394, 1–7. [Google Scholar] [CrossRef]
- Di Gaspero, G.; Radovic, S.; De Luca, E.; Spadotto, A.; Magris, G.; Falginella, L.; Cattonaro, F.; Marroni, F. Evaluation of Sensitivity and Specificity in RNA-Seq-Based Detection of Grapevine Viral Pathogens. J. Virol. Methods 2022, 300, 114383. [Google Scholar] [CrossRef]
- Jalili, V.; Afgan, E.; Gu, Q.; Clements, D.; Blankenberg, D.; Goecks, J.; Taylor, J.; Nekrutenko, A. The Galaxy Platform for Accessible, Reproducible and Collaborative Biomedical Analyses: 2020 Update. Nucleic Acids Res. 2021, 48, W395–W402. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT Online Service: Multiple Sequence Alignment, Interactive Sequence Choice and Visualization. Brief. Bioinform. 2018, 20, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Martin, D.P.; Murrell, B.; Golden, M.; Khoosal, A.; Muhire, B. RDP4: Detection and Analysis of Recombination Patterns in Virus Genomes. Virus Evol. 2015, 1, vev003. [Google Scholar] [CrossRef]
- Ben Mansour, K.; Gibbs, A.J.; Komínková, M.; Komínek, P.; Brožová, J.; Kazda, J.; Zouhar, M.; Ryšánek, P. Watermelon Mosaic Virus in the Czech Republic, Its Recent and Historical Origins. Plant Pathol. 2023, 72, 1528–1538. [Google Scholar] [CrossRef]
- Muhire, B.M.; Varsani, A.; Martin, D.P. SDT: A Virus Classification Tool Based on Pairwise Sequence Alignment and Identity Calculation. PLoS ONE 2014, 9, e108277. [Google Scholar] [CrossRef]
- Fiore, N.; Zamorano, A.; Sánchez-Diana, N.; González, X.; Pallás, V.; Sánchez-Navarro, J. First Detection of Grapevine Rupestris Stem Pitting-Associated Virus and Grapevine Rupestris Vein Feathering Virus, and New Phylogenetic Groups for Grapevine Fleck Virus and Hop Stunt Viroid Isolates, Revealed from Grapevine Field Surveys in Spain. Phytopathol. Mediterr. 2016, 55, 225–238. [Google Scholar]
- Glasa, M.; Predajňa, L.; Šoltys, K.; Sihelská, N.; Nagyová, A.; Wetzel, T.; Sabanadzovic, S. Analysis of Grapevine Rupestris Stem Pitting-Associated Virus in Slovakia Reveals Differences in Intra-Host Population Diversity and Naturally Occurring Recombination Events. Plant Pathol. J. 2017, 33, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Nakaune, R.; Inoue, K.; Nasu, H.; Kakogawa, K.; Nitta, H.; Imada, J.; Nakano, M. Detection of Viruses Associated with Rugose Wood in Japanese Grapevines and Analysis of Genomic Variability of Rupestris Stem Pitting-Associated Virus. J. Gen. Plant Pathol. 2008, 74, 156–163. [Google Scholar] [CrossRef]
- Komínek, P.; Glasa, M.; Bryxiová, M. Analysis of the Molecular Variability of Grapevine Leafroll-Associated Virus 1 Reveals the Presence of Two Distinct Virus Groups and Their Mixed Occurrence. Virus Genes 2005, 31, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Glasa, M.; Predajňa, L.; Komínek, P. Grapevine Fleck Virus Isolates Split into Two Distinct Molecular Groups. J. Phytopathol. 2011, 159, 805–807. [Google Scholar] [CrossRef]
- Sabanadzovic, S.; Abou Ghanem-Sabanadzovic, N.; Saldarelli, P.; Martelli, G.P. Complete Nucleotide Sequence and Genome Organization of Grapevine Fleck Virus. J. Gen. Virol. 2011, 156, 875–879. [Google Scholar] [CrossRef]
- Xiao, H.; Meng, B. First Report of Grapevine Asteroid Mosaic Associated Virus and Grapevine Rupestris Vein Feathering Virus in Grapevines in Canada. Plant Dis. 2016, 100, 2175. [Google Scholar] [CrossRef]
- Jo, Y.; Choi, H.; Kyong Cho, J.; Yoon, J.Y.; Choi, S.K.; Kyong Cho, W. In Silico Approach to Reveal Viral Populations in Grapevine Cultivar Tannat Using Transcriptome Data. Sci. Rep. 2015, 5, 15841. [Google Scholar] [CrossRef] [PubMed]
- Candresse, T.; Faure, C.; Theil, S.; Beuve, M.; Lemaire, O.; Spilmont, A.S.; Marais, A. First Report of Grapevine Asteroid Mosaic-Associated Virus Infecting Grapevine (Vitis vinifera) in France. Plant Dis. 2017, 101, 1061. [Google Scholar] [CrossRef]
- Morán, F.; Canales, C.; Olmos, A.; Ruiz-García, A. First Report of Grapevine Asteroid Mosaic Associated Virus in Grapevine in Spain. Plant Dis. 2020, 105, 517. [Google Scholar] [CrossRef]
- Porceddu, A.; Sanna, M.; Prota, V.A.; Schianchi, N.; Mercenaro, L.; Nieddu, G.; Camiolo, S. First Report of Grapevine Asteroid Mosaic-Associated Virus Infecting Grapevines (Vitis vinifera) in Italy. Plant Dis. 2018, 102, 2049. [Google Scholar] [CrossRef]
- Gibbs, A.J.; Hajizadeh, M.; Ohshima, K.; Jones, R.A.C. The Potyviruses: An Evolutionary Synthesis Is Emerging. Viruses 2020, 12, 132. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Leal, R.; Duffy, S.; Xiong, Z.; Hammond, R.W.; Elena, S.F. Advances in Plant Virus Evolution: Translating Evolutionary Insights into Better Disease Management. Phytopathology 2011, 101, 1136–1148. [Google Scholar] [CrossRef] [PubMed]
- Hily, J.M.; Poulicard, N.; Candresse, T.; Vigne, E.; Beuve, M.; Renault, L.; Velt, A.; Spilmont, A.S.; Lemaire, O. Datamining, Genetic Diversity Analyses, and Phylogeographic Reconstructions Redefine the Worldwide Evolutionary History of Grapevine Pinot Gris Virus and Grapevine Berry Inner Necrosis Virus. Phytobiomes J. 2020, 4, 165–177. [Google Scholar] [CrossRef]
- Cretazzo, E.; Velasco, L. High-throughput Sequencing Allowed the Completion of the Genome of Grapevine Red Globe. Plant Pathol. 2016, 66, 1202–1213. [Google Scholar] [CrossRef]
- Zakubanskiy, A.; Mitrofanova, I.; Sheveleva, A.; Chirkov, S. Analysis of Canna Yellow Streak Virus Complete Genomes Provides Evidence of Multiple Intraspecies Recombination Events. J. Plant Pathol. 2018, 100, 575–580. [Google Scholar] [CrossRef]
- Komínek, P.; Glasa, M.; Komínková, M. Analysis of Multiple Virus-Infected Grapevine Plant Reveals Persistence but Uneven Virus Distribution. Acta Virol. 2009, 53, 281–285. [Google Scholar] [CrossRef]
- Wang, Y.M.; Ostendorf, B.; Pagay, V. Evaluating the Potential of High-Resolution Visible Remote Sensing to Detect Shiraz Disease in Grapevines. Aust. J. Grape Wine Res. 2023, 2023, 7376153. [Google Scholar] [CrossRef]
- Wu, Q.; Habili, N.; Kinoti, W.M.; Tyerman, S.D.; Rinaldo, A.; Zheng, L.; Constable, F.E. A Metagenomic Investigation of the Viruses Associated with Shiraz Disease in Australia. Viruses 2023, 15, 774. [Google Scholar] [CrossRef]






| Grapevine Plant | Cultivar | Origin | Locality |
|---|---|---|---|
| TI23 | Rootstock Kober 125AA | Collection of plant viruses VURV-V | Prague–Ruzyně, district Prague-city |
| KA1 | Müller-Thurgau, clone MT25/7 | Prebasic propagation material | Karlštejn, district Beroun |
| KA3 | Müller-Thurgau, clone MT30/34 | Prebasic propagation material | Karlštejn, district Beroun |
| KA7 | Müller-Thurgau, clone MT25/7 | Prebasic propagation material | Karlštejn, district Beroun |
| KA8 | Traminer | Basic propagation material | Karlštejn, district Beroun |
| LAM3 | Blauer Portugieser | Vineyard survey | Lampelberg, Vrbovec, district Znojmo |
| LAM8 | Grüner Veltliner | Vineyard survey | Lampelberg, Vrbovec, district Znojmo |
| BLA1 | Riesling | Vineyard survey | Blatnice, district Hodonín |
| BLA2 | Grüner Veltliner | Vineyard survey | Blatnice, district Hodonín |
| TVR11 | Blaufränkisch | Vineyard survey | Tvrdonice, district Břeclav |
| LAN21 | Müller-Thurgau | Vineyard survey | Lanžhot, district Břeclav |
| LUZ5 | Sauvignon | Vineyard survey | Lužice, district Hodonín |
| Plant\Virus | GFkV | GRGV | GRVFV | GSyV-1 | GAMaV |
|---|---|---|---|---|---|
| TI23 | 2 | 6 | 2 | ||
| KA1 | 8 | 2 | |||
| KA3 | 4 | 6 | |||
| KA7 | 8 | 2 | |||
| KA8 | 10 | ||||
| LAM3 | 5 | 5 | |||
| LAM8 | 2 | 8 | |||
| BLA1 | 5 | 2 | 3 | ||
| BLA2 | 10 | ||||
| TVR11 | 10 | ||||
| LAN21 | 10 | ||||
| LUZ5 | 10 |
| Plant | Unique Reads in Total | Virus/Viroid Detected | Unique Reads Mapped to Virus/Viroid | Genome Coverage (%) |
|---|---|---|---|---|
| TI23 | 277,961 | GVA | 188 | 41.5 |
| GVB | 38 | 23.7 | ||
| GRSPaV-1 | 161 | 61.7 | ||
| GLRaV-1 | 1357 | 98.8 | ||
| HSVd | 36 | 100 | ||
| LAM3 | 908,759 | GFkV | 422 | 92.8 |
| GRSPaV-1 | 952 | 99.99 | ||
| GYSVd-1 | 62 | 100 | ||
| HSVd | 75 | 100 | ||
| LAM8 | 328,278 | GFkV | 422 | 92.8 |
| GRSPaV-1 | 1135 | 99.9 | ||
| GYSVd-1 | 34 | 100 | ||
| HSVd | 46 | 100 | ||
| BLA1 | 186,218 | GSyV-1 | 1 | 2.2 |
| GPGV | 21 | 16.8 | ||
| GRSPaV-1 | 58 | 68.5 | ||
| GYSVd-1 | 66 | 100 | ||
| HSVd | 54 | 100 |
| Plant | Unique Reads in Total | Virus/Viroid Detected | Unique Reads Assigned to Virus | Genome Coverage (%) |
|---|---|---|---|---|
| BLA1 | 9,590,966 | GRGV | 41 | 29.8 |
| GRVFV | 9 | 9.4 | ||
| GSyV-1 | 47 | 32.3 | ||
| GPGV | 265 | 81.8 | ||
| GRSPaV-1 | 1705 | 99.7 | ||
| GYSVd-1 | 293 | 100 | ||
| HSVd | 670 | 100 |
| Virus Isolate | GenBank Acc. No. | Sequence Length | Reference Sequence for Mapping | Reference Length | Pairwise Nucleotide Identity with Reference Sequence (%) | Coverage of Reference Sequence (%) | No. of Reads Mapped to the Reference Sequence Using Galaxy | No. of Reads Mapped to the Reference Sequence Using Geneious |
|---|---|---|---|---|---|---|---|---|
| GRGV-1 | OR787584 | 6849 | MZ451070 | 6850 | 96.92 | 100.00 | 287,329 | 502,284 |
| GRGV-2 | OR787585 | 6837 | KX171167 | 6851 | 97.18 | 100.00 | 352,316 | 413,327 |
| GSyV-1-3 | OR787586 | 6481 | FJ436028 | 6506 | 87.09 | 100.00 | 392,806 | 209,449 |
| GSyV-1-4 | OR787587 | 6482 | KP221255 | 6482 | 96.7 | 100.00 | 383,796 | 311,300 |
| GRVFV-5 | OR787588 | 6588 | MZ451085 | 6718 | 85.68 | 100.00 | 539,512 | 995,741 |
| GRVFV-6 | OR787589 | 6727 | MT084814 | 6718 | 96.53 | 100.00 | 400,088 | 477,244 |
| Italian NC_003347 | 5’UTR | Rep | CP | ORF3 | ORF4 | 3′UTR | ||||
| nt | nt | aa | nt | aa | nt | aa | nt | aa | nt | |
| Czech OR701334 | 91.7 | 91.4 | 95.8 | 95.5 | 99.1 | 94.4 | 88.3 | 94.3 | 87.2 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komínková, M.; Ben Mansour, K.; Komínek, P.; Brožová, J.; Střalková, R. Multiple Infections with Viruses of the Family Tymoviridae in Czech Grapevines. Viruses 2024, 16, 343. https://doi.org/10.3390/v16030343
Komínková M, Ben Mansour K, Komínek P, Brožová J, Střalková R. Multiple Infections with Viruses of the Family Tymoviridae in Czech Grapevines. Viruses. 2024; 16(3):343. https://doi.org/10.3390/v16030343
Chicago/Turabian StyleKomínková, Marcela, Karima Ben Mansour, Petr Komínek, Jana Brožová, and Radomíra Střalková. 2024. "Multiple Infections with Viruses of the Family Tymoviridae in Czech Grapevines" Viruses 16, no. 3: 343. https://doi.org/10.3390/v16030343