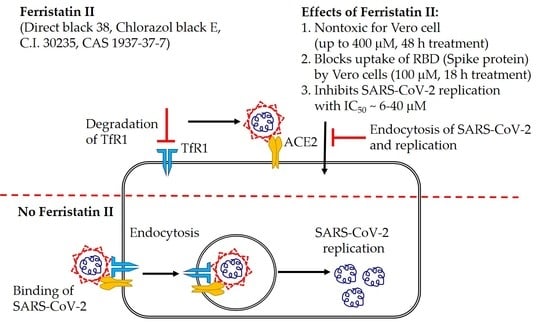
Ferristatin II Efficiently Inhibits SARS-CoV-2 Replication in Vero Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Viruses, Proteins and Chemical Compounds
2.2. SARS-CoV-2 Virus Propagation and the Assessment of Ferristatin II Virus Inhibition Capacity
2.3. Assessment of Cytotoxic Effect of Ferristatin II
2.4. Labeling of Human Serum Transferrin and Recombinant RBD Protein
2.5. Study of Ferristatin II Effect of TF and RBD Uptake by Vero Cells
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scialo, F.; Daniele, A.; Amato, F.; Pastore, L.; Matera, M.G.; Cazzola, M.; Castaldo, G.; Bianco, A. ACE2: The Major Cell Entry Receptor for SARS-CoV-2. Lung 2020, 198, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Yang, X.; Yang, D.; Bao, J.; Li, R.; Xiao, Y.; Hou, C.; Wang, H.; Liu, J.; Yang, D.; et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit. Care 2020, 24, 422. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Ye, G.; Shi, K.; Wan, Y.; Luo, C.; Aihara, H.; Geng, Q.; Auerbach, A.; Li, F. Structural basis of receptor recognition by SARS-CoV-2. Nature 2020, 581, 221–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, Y.; Shang, J.; Graham, R.; Baric, R.S.; Li, F. Receptor Recognition by the Novel Coronavirus from Wuhan: An Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J. Virol. 2020, 94, e00127-20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Chen, C.Z.; Swaroop, M.; Xu, M.; Wang, L.; Lee, J.; Wang, A.Q.; Pradhan, M.; Hagen, N.; Chen, L.; et al. Heparan sulfate assists SARS-CoV-2 in cell entry and can be targeted by approved drugs in vitro. Cell Discov. 2020, 6, 80. [Google Scholar] [CrossRef]
- Wang, K.; Chen, W.; Zhang, Z.; Deng, Y.; Lian, J.Q.; Du, P.; Wei, D.; Zhang, Y.; Sun, X.X.; Gong, L.; et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct. Target. Ther. 2020, 5, 283. [Google Scholar] [CrossRef]
- Wei, C.; Wan, L.; Yan, Q.; Wang, X.; Zhang, J.; Yang, X.; Zhang, Y.; Fan, C.; Li, D.; Deng, Y.; et al. HDL-scavenger receptor B type 1 facilitates SARS-CoV-2 entry. Nat. Metab. 2020, 2, 1391–1400. [Google Scholar] [CrossRef]
- Daly, J.L.; Simonetti, B.; Klein, K.; Chen, K.E.; Williamson, M.K.; Anton-Plagaro, C.; Shoemark, D.K.; Simon-Gracia, L.; Bauer, M.; Hollandi, R.; et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science 2020, 370, 861–865. [Google Scholar] [CrossRef]
- Wang, S.; Qiu, Z.; Hou, Y.; Deng, X.; Xu, W.; Zheng, T.; Wu, P.; Xie, S.; Bian, W.; Zhang, C.; et al. AXL is a candidate receptor for SARS-CoV-2 that promotes infection of pulmonary and bronchial epithelial cells. Cell Res. 2021, 31, 126–140. [Google Scholar] [CrossRef]
- Cantuti-Castelvetri, L.; Ojha, R.; Pedro, L.D.; Djannatian, M.; Franz, J.; Kuivanen, S.; van der Meer, F.; Kallio, K.; Kaya, T.; Anastasina, M.; et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 2020, 370, 856–860. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Yang, M.; Duan, Z.; Liao, Z.; Liu, L.; Cheng, R.; Fang, M.; Wang, G.; Liu, H.; Xu, J.; et al. Transferrin receptor is another receptor for SARS-CoV-2 entry. bioRxiv 2020. [Google Scholar] [CrossRef]
- Hentze, M.W.; Muckenthaler, M.U.; Andrews, N.C. Balancing acts: Molecular control of mammalian iron metabolism. Cell 2004, 117, 285–297. [Google Scholar] [CrossRef] [Green Version]
- Wessling-Resnick, M. Crossing the Iron Gate: Why and How Transferrin Receptors Mediate Viral Entry. Annu. Rev. Nutr. 2018, 38, 431–458. [Google Scholar] [CrossRef] [PubMed]
- Horonchik, L.; Wessling-Resnick, M. The small-molecule iron transport inhibitor ferristatin/NSC306711 promotes degradation of the transferrin receptor. Chem. Biol. 2008, 15, 647–653. [Google Scholar] [CrossRef] [Green Version]
- Byrne, S.L.; Buckett, P.D.; Kim, J.; Luo, F.; Sanford, J.; Chen, J.; Enns, C.; Wessling-Resnick, M. Ferristatin II promotes degradation of transferrin receptor-1 in vitro and in vivo. PLoS ONE 2013, 8, e70199. [Google Scholar] [CrossRef] [Green Version]
- Sokolov, A.V.; Voynova, I.V.; Kostevich, V.A.; Vlasenko, A.Y.; Zakharova, E.T.; Vasilyev, V.B. Comparison of Interaction between Ceruloplasmin and Lactoferrin/Transferrin: To Bind or Not to Bind. Biochem. Biokhimiia 2017, 82, 1073–1078. [Google Scholar] [CrossRef]
- Amanat, F.; White, K.M.; Miorin, L.; Strohmeier, S.; McMahon, M.; Meade, P.; Liu, W.C.; Albrecht, R.A.; Simon, V.; Martinez-Sobrido, L.; et al. An In Vitro Microneutralization Assay for SARS-CoV-2 Serology and Drug Screening. Curr. Protoc. Microbiol. 2020, 58, e108. [Google Scholar] [CrossRef]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef]
- Borenfreund, E.; Babich, H.; Martin-Alguacil, N. Comparisons of two in vitro cytotoxicity assays-The neutral red (NR) and tetrazolium MTT tests. Toxicol. In Vitro 1988, 2, 1–6. [Google Scholar] [CrossRef]
- Xiu, S.; Dick, A.; Ju, H.; Mirzaie, S.; Abdi, F.; Cocklin, S.; Zhan, P.; Liu, X. Inhibitors of SARS-CoV-2 Entry: Current and Future Opportunities. J. Med. Chem. 2020, 63, 12256–12274. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.X.; Buckett, P.D.; Wessling-Resnick, M. Identification of small molecule inhibitors that distinguish between non-transferrin bound iron uptake and transferrin-mediated iron transport. Chem. Biol. 2004, 11, 407–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, D.N.; Uprichard, S.L. Identification of transferrin receptor 1 as a hepatitis C virus entry factor. Proc. Natl. Acad. Sci. USA 2013, 110, 10777–10782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barretto, N.; Sainz, B., Jr.; Hussain, S.; Uprichard, S.L. Determining the involvement and therapeutic implications of host cellular factors in hepatitis C virus cell-to-cell spread. J. Virol. 2014, 88, 5050–5061. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Hu, W.; Yuan, L.; Yang, Q. Transferrin receptor 1 is a supplementary receptor that assists transmissible gastroenteritis virus entry into porcine intestinal epithelium. Cell Commun. Signal. CCS 2018, 16, 69. [Google Scholar] [CrossRef] [Green Version]
- Wan, Q.; Liao, Z.; Rao, Y.; Yang, C.; Ji, J.; Chen, X.; Su, J. Transferrin Receptor 1-Associated Iron Accumulation and Oxidative Stress Provides a Way for Grass Carp to Fight against Reovirus Infection. Int. J. Mol. Sci. 2019, 20, 5857. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Cao, Y.; Yang, Q. Transferrin receptor 1 levels at the cell surface influence the susceptibility of newborn piglets to PEDV infection. PLoS Pathog. 2020, 16, e1008682. [Google Scholar] [CrossRef]
- Prabhakara, C.; Godbole, R.; Sil, P.; Jahnavi, S.; Gulzar, S.E.; van Zanten, T.S.; Sheth, D.; Subhash, N.; Chandra, A.; Shivaraj, A.; et al. Strategies to target SARS-CoV-2 entry and infection using dual mechanisms of inhibition by acidification inhibitors. PLoS Pathog. 2021, 17, e1009706. [Google Scholar] [CrossRef]
- Puray-Chavez, M.; LaPak, K.M.; Schrank, T.P.; Elliott, J.L.; Bhatt, D.P.; Agajanian, M.J.; Jasuja, R.; Lawson, D.Q.; Davis, K.; Rothlauf, P.W.; et al. Systematic analysis of SARS-CoV-2 infection of an ACE2-negative human airway cell. Cell Rep. 2021, 36, 109364. [Google Scholar] [CrossRef]
- Alkhateeb, A.A.; Buckett, P.D.; Gardeck, A.M.; Kim, J.; Byrne, S.L.; Fraenkel, P.G.; Wessling-Resnick, M. The small molecule ferristatin II induces hepatic hepcidin expression in vivo and in vitro. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G1019–G1026. [Google Scholar] [CrossRef] [Green Version]
- Romer, M.; Eichner, J.; Metzger, U.; Templin, M.F.; Plummer, S.; Ellinger-Ziegelbauer, H.; Zell, A. Cross-platform toxicogenomics for the prediction of non-genotoxic hepatocarcinogenesis in rat. PLoS ONE 2014, 9, e97640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kass, L. Staining of granulocytic cells by Chlorazol black E. Am. J. Clin. Pathol. 1981, 76, 810–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burke, W.A.; Jones, B.E. A simple stain for rapid office diagnosis of fungus infections of the skin. Arch. Dermatol. 1984, 120, 1519–1520. [Google Scholar] [CrossRef]
- Dewan, A.; Jani, J.P.; Patel, J.S.; Gandhi, D.N.; Variya, M.R.; Ghodasara, N.B. Benzidine and its acetylated metabolites in the urine of workers exposed to Direct Black 38. Arch. Environ. Health 1988, 43, 269–272. [Google Scholar] [CrossRef] [PubMed]





| Virus | Ferristatin Dose, µM | Time of Treatment | Effect | Reference |
|---|---|---|---|---|
| Hepatitis C virus (HCV) | 25–50 | 20 h | Significant reduction in HCV cell-to-cell spread observed in ferristatin-treated Huh7 cells | [23] |
| Transmissible gastroenteritis virus (TGEV) | 50 | 24 h | 3-fold reduction in viral TGEV-N protein level in IPEC-J2 cells | [25] |
| Porcine epidemic diarrhea virus (PEDV) | 50 | 3h | 3-fold reduction in PEDV titer and RNA level in IPEC-J2 and Vero cells | [27] |
| Glass carp reovirus (GCRV) | 100 | 24 h | (2–3)-fold increase in expression of interferons (IFN-1 and IFN-3) in GCRV-infected CIK cells | [26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokolov, A.; Isakova-Sivak, I.; Grudinina, N.; Mezhenskaya, D.; Litasova, E.; Kostevich, V.; Stepanova, E.; Rak, A.; Sychev, I.; Kirik, O.; et al. Ferristatin II Efficiently Inhibits SARS-CoV-2 Replication in Vero Cells. Viruses 2022, 14, 317. https://doi.org/10.3390/v14020317
Sokolov A, Isakova-Sivak I, Grudinina N, Mezhenskaya D, Litasova E, Kostevich V, Stepanova E, Rak A, Sychev I, Kirik O, et al. Ferristatin II Efficiently Inhibits SARS-CoV-2 Replication in Vero Cells. Viruses. 2022; 14(2):317. https://doi.org/10.3390/v14020317
Chicago/Turabian StyleSokolov, Alexey, Irina Isakova-Sivak, Natalia Grudinina, Daria Mezhenskaya, Elena Litasova, Valeria Kostevich, Ekaterina Stepanova, Alexandra Rak, Ivan Sychev, Olga Kirik, and et al. 2022. "Ferristatin II Efficiently Inhibits SARS-CoV-2 Replication in Vero Cells" Viruses 14, no. 2: 317. https://doi.org/10.3390/v14020317