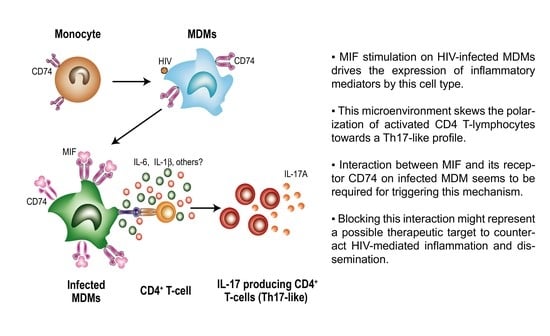
Macrophage Migration Inhibitory Factor (MIF) Promotes Increased Proportions of the Highly Permissive Th17-like Cell Profile during HIV Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Primary Human Monocyte-Derived Macrophages (MDMs) and CD4+ T-Lymphocyte (CD4TL) Purification and Culture
2.2. Virus Production and Infections
2.3. MDM/CD4TL Co-cultures
2.4. Recombinant Cytokines and Antibodies
2.5. Cytokine Quantitation
2.6. CD4TL Phenotype, Viability and Infection Percentage
2.7. Quantitative Real-Time PCR for Cell-Associated (CA) HIV DNA
2.8. Naïve and Memory CD4TL Cell Sorting
2.9. Human Samples from People with HIV
2.10. Data Analysis
3. Results
3.1. Cytokine Expression by MIF-Treated HIV-Infected MDMs
3.2. CD4TL Differentiation and Activation Profile after Contact with MIF-Treated HIV-Infected MDMs
3.3. CD4TL Trans-Infection by MIF-Treated HIV-Infected MDMs
3.4. Soluble Factors Released after MIF Treatment in HIV-Infected MDMs Implicated in CD4TL Polarization
3.5. MDMs Infected with T/F HIV Strains Also Promote the Generation of a Th17-like Profile after MIF Treatment
3.6. Profiling of Naïve and Memory CD4TLs by the Environment of HIV-Infected MIF-Treated MDMs
3.7. Ex Vivo Association between MIF and Th17-like Cells in HIV Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maartens, G.; Celum, C.; Lewin, S.R. HIV infection: Epidemiology, pathogenesis, treatment, and prevention. Lancet 2014, 384, 258–271. [Google Scholar] [CrossRef]
- Okoli, C.; Van de Velde, N.; Richman, B.; Allan, B.; Castellanos, E.; Young, B.; Brough, G.; Eremin, A.; Corbelli, G.M.; Mc Britton, M.; et al. Undetectable equals untransmittable (U = U): Awareness and associations with health outcomes among people living with HIV in 25 countries. Sex. Transm. Infect. 2021, 97, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Deeks, S.G.; Tracy, R.; Douek, D.C. Systemic effects of inflammation on health during chronic HIV infection. Immunity 2013, 39, 633–645. [Google Scholar] [CrossRef] [Green Version]
- Lv, T.; Cao, W.; Li, T. HIV-Related Immune Activation and Inflammation: Current Understanding and Strategies. J. Immunol. Res. 2021, 2021, 7316456. [Google Scholar] [CrossRef] [PubMed]
- Fromentin, R.; Chomont, N. HIV persistence in subsets of CD4+ T cells: 50 shades of reservoirs. Semin. Immunol. 2021, 51, 101438. [Google Scholar] [CrossRef] [PubMed]
- Deeks, S.G.; Archin, N.; Cannon, P.; Collins, S.; Jones, R.B.; de Jong, M.; Lambotte, O.; Lamplough, R.; Ndung’u, T.; Sugarman, J.; et al. Research priorities for an HIV cure: International AIDS Society Global Scientific Strategy 2021. Nat. Med. 2021, 27, 2085–2098. [Google Scholar] [CrossRef]
- Quaresma, J.A.S. Organization of the Skin Immune System and Compartmentalized Immune Responses in Infectious Diseases. Clin. Microbiol. Rev. 2019, 32, e00034-18. [Google Scholar] [CrossRef]
- Orlova-Fink, N.; Chowdhury, F.Z.; Sun, X.; Harrington, S.; Rosenberg, E.S.; Yu, X.G.; Lichterfeld, M. Preferential susceptibility of Th9 and Th2 CD4+ T cells to X4-tropic HIV-1 infection. Aids 2017, 31, 2211–2215. [Google Scholar] [CrossRef]
- Sun, H.; Kim, D.; Li, X.; Kiselinova, M.; Ouyang, Z.; Vandekerckhove, L.; Shang, H.; Rosenberg, E.S.; Yu, X.G.; Lichterfeld, M. Th1/17 Polarization of CD4 T Cells Supports HIV-1 Persistence during Antiretroviral Therapy. J. Virol. 2015, 89, 11284–11293. [Google Scholar] [CrossRef] [Green Version]
- Mensching, L.; Hoelzemer, A. NK Cells, Monocytes and Macrophages in HIV-1 Control: Impact of Innate Immune Responses. Front. Immunol. 2022, 13, 883728. [Google Scholar] [CrossRef]
- Pagani, I.; Demela, P.; Ghezzi, S.; Vicenzi, E.; Pizzato, M.; Poli, G. Host Restriction Factors Modulating HIV Latency and Replication in Macrophages. Int. J. Mol. Sci. 2022, 23, 3021. [Google Scholar] [CrossRef] [PubMed]
- Bilsborrow, J.B.; Doherty, E.; Tilstam, P.V.; Bucala, R. Macrophage migration inhibitory factor (MIF) as a therapeutic target for rheumatoid arthritis and systemic lupus erythematosus. Expert Opin. Ther. Targets 2019, 23, 733–744. [Google Scholar] [CrossRef]
- Kang, I.; Bucala, R. The immunobiology of MIF: Function, genetics and prospects for precision medicine. Nat. Rev. Rheumatol. 2019, 15, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Delaloye, J.; De Bruin, I.J.; Darling, K.E.; Reymond, M.K.; Sweep, F.C.; Roger, T.; Calandra, T.; Cavassini, M. Increased macrophage migration inhibitory factor (MIF) plasma levels in acute HIV-1 infection. Cytokine 2012, 60, 338–340. [Google Scholar] [CrossRef] [PubMed]
- Regis, E.G.; Barreto-de-Souza, V.; Morgado, M.G.; Bozza, M.T.; Leng, L.; Bucala, R.; Bou-Habib, D.C. Elevated levels of macrophage migration inhibitory factor (MIF) in the plasma of HIV-1-infected patients and in HIV-1-infected cell cultures: A relevant role on viral replication. Virology 2010, 399, 31–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trifone, C.; Salido, J.; Ruiz, M.J.; Leng, L.; Quiroga, M.F.; Salomon, H.; Bucala, R.; Ghiglione, Y.; Turk, G. Interaction Between Macrophage Migration Inhibitory Factor and CD74 in Human Immunodeficiency Virus Type I Infected Primary Monocyte-Derived Macrophages Triggers the Production of Proinflammatory Mediators and Enhances Infection of Unactivated CD4(+) T Cells. Front. Immunol. 2018, 9, 1494. [Google Scholar] [PubMed] [Green Version]
- Bernhagen, J.; Krohn, R.; Lue, H.; Gregory, J.L.; Zernecke, A.; Koenen, R.R.; Dewor, M.; Georgiev, I.; Schober, A.; Leng, L.; et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat. Med. 2007, 13, 587–596. [Google Scholar] [CrossRef]
- Leng, L.; Metz, C.N.; Fang, Y.; Xu, J.; Donnelly, S.; Baugh, J.; Delohery, T.; Chen, Y.; Mitchell, R.A.; Bucala, R. MIF signal transduction initiated by binding to CD74. J. Exp. Med. 2003, 197, 1467–1476. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Leng, L.; Wang, T.; Wang, W.; Du, X.; Li, J.; McDonald, C.; Chen, Z.; Murphy, J.W.; Lolis, E.; et al. CD44 is the signaling component of the macrophage migration inhibitory factor-CD74 receptor complex. Immunity 2006, 25, 595–606. [Google Scholar] [CrossRef] [Green Version]
- Dugast, M.; Toussaint, H.; Dousset, C.; Benaroch, P. AP2 clathrin adaptor complex, but not AP1, controls the access of the major histocompatibility complex (MHC) class II to endosomes. J. Biol. Chem. 2005, 280, 19656–19664. [Google Scholar] [CrossRef]
- Ghiglione, Y.; Rodriguez, A.M.; De Candia, C.; Carobene, M.; Benaroch, P.; Schindler, M.; Salomon, H.; Turk, G. HIV-mediated up-regulation of invariant chain (CD74) correlates with generalized immune activation in HIV+ subjects. Virus Res. 2012, 163, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Keele, B.F.; Giorgi, E.E.; Salazar-Gonzalez, J.F.; Decker, J.M.; Pham, K.T.; Salazar, M.G.; Sun, C.; Grayson, T.; Wang, S.; Li, H.; et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl. Acad. Sci. USA 2008, 105, 7552–7557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.Y.; Giorgi, E.E.; Keele, B.F.; Gaschen, B.; Athreya, G.S.; Salazar-Gonzalez, J.F.; Pham, K.T.; Goepfert, P.A.; Kilby, J.M.; Saag, M.S.; et al. Modeling sequence evolution in acute HIV-1 infection. J. Theor. Biol. 2009, 261, 341–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernhagen, J.; Mitchell, R.A.; Calandra, T.; Voelter, W.; Cerami, A.; Bucala, R. Purification, bioactivity, and secondary structure analysis of mouse and human macrophage migration inhibitory factor (MIF). Biochemistry 1994, 33, 14144–14155. [Google Scholar] [CrossRef] [PubMed]
- Hare, A.A.; Leng, L.; Gandavadi, S.; Du, X.; Cournia, Z.; Bucala, R.; Jorgensen, W.L. Optimization of N-benzyl-benzoxazol-2-ones as receptor antagonists of macrophage migration inhibitory factor (MIF). Bioorg. Med. Chem. Lett. 2010, 20, 5811–5814. [Google Scholar] [CrossRef] [Green Version]
- Leng, L.; Chen, L.; Fan, J.; Greven, D.; Arjona, A.; Du, X.; Austin, D.; Kashgarian, M.; Yin, Z.; Huang, X.R.; et al. A small-molecule macrophage migration inhibitory factor antagonist protects against glomerulonephritis in lupus-prone NZB/NZW F1 and MRL/lpr mice. J. Immunol. 2011, 186, 527–538. [Google Scholar] [CrossRef] [Green Version]
- Rowe, M.A.; Harper, L.R.; McNulty, M.A.; Lau, A.G.; Carlson, C.S.; Leng, L.; Bucala, R.J.; Miller, R.A.; Loeser, R.F. Reduced Osteoarthritis Severity in Aged Mice with Deletion of Macrophage Migration Inhibitory Factor. Arthritis Rheumatol. 2017, 69, 352–361. [Google Scholar] [CrossRef] [Green Version]
- Vandergeeten, C.; Fromentin, R.; Merlini, E.; Lawani, M.B.; DaFonseca, S.; Bakeman, W.; McNulty, A.; Ramgopal, M.; Michael, N.; Kim, J.H.; et al. Cross-clade ultrasensitive PCR-based assays to measure HIV persistence in large-cohort studies. J. Virol. 2014, 88, 12385–12396. [Google Scholar] [CrossRef] [Green Version]
- Chomont, N.; El-Far, M.; Ancuta, P.; Trautmann, L.; Procopio, F.A.; Yassine-Diab, B.; Boucher, G.; Boulassel, M.R.; Ghattas, G.; Brenchley, J.M.; et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat. Med. 2009, 15, 893–900. [Google Scholar] [CrossRef]
- Salido, J.; Czernikier, A.; Trifone, C.; Polo, M.L.; Figueroa, M.I.; Urioste, A.; Cahn, P.; Sued, O.; Salomon, H.; Laufer, N.; et al. Pre-cART Immune Parameters in People Living With HIV Might Help Predict CD8+ T-Cell Characteristics, Inflammation Levels, and Reservoir Composition After Effective cART. Pathog. Immun. 2021, 6, 60–89. [Google Scholar] [CrossRef]
- Ghiglione, Y.; Falivene, J.; Ruiz, M.J.; Laufer, N.; Socias, M.E.; Cahn, P.; Giavedoni, L.; Sued, O.; Gherardi, M.M.; Salomon, H.; et al. Early skewed distribution of total and HIV-specific CD8+ T-cell memory phenotypes during primary HIV infection is related to reduced antiviral activity and faster disease progression. PLoS ONE 2014, 9, e104235. [Google Scholar] [CrossRef] [PubMed]
- Ghiglione, Y.; Trifone, C.; Salido, J.; Rhodes, A.; Ruiz, M.J.; Polo, M.L.; Salomon, H.; Laufer, N.; Sued, O.; Lewin, S.R.; et al. PD-1 Expression in HIV-Specific CD8+ T cells Before Antiretroviral Therapy Is Associated with HIV Persistence. J. Acquir. Immune Defic. Syndr. 2019, 80, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.J.; Ghiglione, Y.; Falivene, J.; Laufer, N.; Holgado, M.P.; Socias, M.E.; Cahn, P.; Sued, O.; Giavedoni, L.; Salomon, H.; et al. Env-Specific IgA from Viremic HIV-Infected Subjects Compromises Antibody-Dependent Cellular Cytotoxicity. J. Virol. 2016, 90, 670–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salido, J.; Ruiz, M.J.; Trifone, C.; Figueroa, M.I.; Caruso, M.P.; Gherardi, M.M.; Sued, O.; Salomon, H.; Laufer, N.; Ghiglione, Y.; et al. Phenotype, Polyfunctionality, and Antiviral Activity of in vitro Stimulated CD8(+) T-Cells From HIV(+) Subjects Who Initiated cART at Different Time-Points After Acute Infection. Front. Immunol. 2018, 9, 2443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Socias, M.E.; Sued, O.; Laufer, N.; Lazaro, M.E.; Mingrone, H.; Pryluka, D.; Remondegui, C.; Figueroa, M.I.; Cesar, C.; Gun, A.; et al. Acute retroviral syndrome and high baseline viral load are predictors of rapid HIV progression among untreated Argentinean seroconverters. J. Int. Aids Soc. 2011, 14, 40. [Google Scholar] [CrossRef] [Green Version]
- Turk, G.; Ghiglione, Y.; Falivene, J.; Socias, M.E.; Laufer, N.; Coloccini, R.S.; Rodriguez, A.M.; Ruiz, M.J.; Pando, M.A.; Giavedoni, L.D.; et al. Early Gag immunodominance of the HIV-specific T-cell response during acute/early infection is associated with higher CD8+ T-cell antiviral activity and correlates with preservation of the CD4+ T-cell compartment. J. Virol. 2013, 87, 7445–7462. [Google Scholar] [CrossRef] [Green Version]
- Turk, G.; Ghiglione, Y.; Hormanstorfer, M.; Laufer, N.; Coloccini, R.; Salido, J.; Trifone, C.; Ruiz, M.J.; Falivene, J.; Holgado, M.P.; et al. Biomarkers of Progression after HIV Acute/Early Infection: Nothing Compares to CD4(+) T-cell Count? Viruses 2018, 10, 34. [Google Scholar] [CrossRef] [Green Version]
- Falivene, J.; Ghiglione, Y.; Laufer, N.; Socias, M.E.; Holgado, M.P.; Ruiz, M.J.; Maeto, C.; Figueroa, M.I.; Giavedoni, L.D.; Cahn, P.; et al. Th17 and Th17/Treg ratio at early HIV infection associate with protective HIV-specific CD8(+) T-cell responses and disease progression. Sci. Rep. 2015, 5, 11511. [Google Scholar] [CrossRef] [Green Version]
- Harris, J.; VanPatten, S.; Deen, N.S.; Al-Abed, Y.; Morand, E.F. Rediscovering MIF: New Tricks for an Old Cytokine. Trends Immunol. 2019, 40, 447–462. [Google Scholar] [CrossRef]
- Locati, M.; Curtale, G.; Mantovani, A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu. Rev. Pathol. 2020, 15, 123–147. [Google Scholar] [CrossRef]
- Ivanov, I.I.; McKenzie, B.S.; Zhou, L.; Tadokoro, C.E.; Lepelley, A.; Lafaille, J.J.; Cua, D.J.; Littman, D.R. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 2006, 126, 1121–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De la Cruz-Mosso, U.; Garcia-Iglesias, T.; Bucala, R.; Estrada-Garcia, I.; Gonzalez-Lopez, L.; Cerpa-Cruz, S.; Parra-Rojas, I.; Gamez-Nava, J.I.; Perez-Guerrero, E.E.; Munoz-Valle, J.F. MIF promotes a differential Th1/Th2/Th17 inflammatory response in human primary cell cultures: Predominance of Th17 cytokine profile in PBMC from healthy subjects and increase of IL-6 and TNF-alpha in PBMC from active SLE patients. Cell. Immunol. 2018, 324, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Arellano, S.; Hernandez-Palma, L.A.; Bucala, R.; Hernandez-Bello, J.; De la Cruz-Mosso, U.; Garcia-Iglesias, T.; Cerpa-Cruz, S.; Padilla-Gutierrez, J.R.; Valle, Y.; Sonanez-Organis, J.G.; et al. Th1/Th17 Cytokine Profile is Induced by Macrophage Migration Inhibitory Factor in Peripheral Blood Mononuclear Cells from Rheumatoid Arthritis Patients. Curr. Mol. Med. 2018, 18, 679–688. [Google Scholar] [CrossRef]
- Schindler, M.; Wurfl, S.; Benaroch, P.; Greenough, T.C.; Daniels, R.; Easterbrook, P.; Brenner, M.; Munch, J.; Kirchhoff, F. Down-modulation of mature major histocompatibility complex class II and up-regulation of invariant chain cell surface expression are well-conserved functions of human and simian immunodeficiency virus nef alleles. J. Virol. 2003, 77, 10548–10556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendricks, C.M.; Cordeiro, T.; Gomes, A.P.; Stevenson, M. The Interplay of HIV-1 and Macrophages in Viral Persistence. Front. Microbiol. 2021, 12, 646447. [Google Scholar] [CrossRef]
- Mamik, M.K.; Ghorpade, A. Chemokine CXCL8 promotes HIV-1 replication in human monocyte-derived macrophages and primary microglia via nuclear factor-kappaB pathway. PLoS ONE 2014, 9, e92145. [Google Scholar] [CrossRef] [Green Version]
- Cerboni, S.; Gehrmann, U.; Preite, S.; Mitra, S. Cytokine-regulated Th17 plasticity in human health and diseases. Immunology 2021, 163, 3–18. [Google Scholar] [CrossRef]
- McGeachy, M.J.; Bak-Jensen, K.S.; Chen, Y.; Tato, C.M.; Blumenschein, W.; McClanahan, T.; Cua, D.J. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat. Immunol. 2007, 8, 1390–1397. [Google Scholar] [CrossRef]
- Kimura, A.; Kishimoto, T. IL-6: Regulator of Treg/Th17 balance. Eur. J. Immunol. 2010, 40, 1830–1835. [Google Scholar] [CrossRef]
- Heinrich, P.C.; Behrmann, I.; Haan, S.; Hermanns, H.M.; Muller-Newen, G.; Schaper, F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003, 374, 1–20. [Google Scholar] [CrossRef]
- Flynn, C.M.; Kespohl, B.; Daunke, T.; Garbers, Y.; Dusterhoft, S.; Rose-John, S.; Haybaeck, J.; Lokau, J.; Aparicio-Siegmund, S.; Garbers, C. Interleukin-6 controls recycling and degradation, but not internalization of its receptors. J. Biol. Chem. 2021, 296, 100434. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.R.; Berthoud, T.K.; Kumar, A.; Angel, J.B. IL-23 signaling in Th17 cells is inhibited by HIV infection and is not restored by HAART: Implications for persistent immune activation. PLoS ONE 2017, 12, e0186823. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, I.; Cvjeticanin, T.; Lazaroski, S.; Stosic-Grujicic, S.; Miljkovic, D. Macrophage migration inhibitory factor stimulates interleukin-17 expression and production in lymph node cells. Immunology 2009, 126, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Clayton, K.L.; Garcia, J.V.; Clements, J.E.; Walker, B.D. HIV Infection of Macrophages: Implications for Pathogenesis and Cure. Pathog. Immun. 2017, 2, 179–192. [Google Scholar] [CrossRef] [Green Version]
- Brenchley, J.M.; Paiardini, M.; Knox, K.S.; Asher, A.I.; Cervasi, B.; Asher, T.E.; Scheinberg, P.; Price, D.A.; Hage, C.A.; Kholi, L.M.; et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood 2008, 112, 2826–2835. [Google Scholar] [CrossRef] [Green Version]
- Prendergast, A.; Prado, J.G.; Kang, Y.H.; Chen, F.; Riddell, L.A.; Luzzi, G.; Goulder, P.; Klenerman, P. HIV-1 infection is characterized by profound depletion of CD161+ Th17 cells and gradual decline in regulatory T cells. Aids 2010, 24, 491–502. [Google Scholar] [CrossRef]
- Tuzlak, S.; Dejean, A.S.; Iannacone, M.; Quintana, F.J.; Waisman, A.; Ginhoux, F.; Korn, T.; Becher, B. Repositioning TH cell polarization from single cytokines to complex help. Nat. Immunol. 2021, 22, 1210–1217. [Google Scholar] [CrossRef]
- Lee, G.R. The Balance of Th17 versus Treg Cells in Autoimmunity. Int. J. Mol. Sci. 2018, 19, 730. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trifone, C.; Baquero, L.; Czernikier, A.; Benencio, P.; Leng, L.; Laufer, N.; Quiroga, M.F.; Bucala, R.; Ghiglione, Y.; Turk, G. Macrophage Migration Inhibitory Factor (MIF) Promotes Increased Proportions of the Highly Permissive Th17-like Cell Profile during HIV Infection. Viruses 2022, 14, 2218. https://doi.org/10.3390/v14102218
Trifone C, Baquero L, Czernikier A, Benencio P, Leng L, Laufer N, Quiroga MF, Bucala R, Ghiglione Y, Turk G. Macrophage Migration Inhibitory Factor (MIF) Promotes Increased Proportions of the Highly Permissive Th17-like Cell Profile during HIV Infection. Viruses. 2022; 14(10):2218. https://doi.org/10.3390/v14102218
Chicago/Turabian StyleTrifone, César, Lucía Baquero, Alejandro Czernikier, Paula Benencio, Lin Leng, Natalia Laufer, María Florencia Quiroga, Richard Bucala, Yanina Ghiglione, and Gabriela Turk. 2022. "Macrophage Migration Inhibitory Factor (MIF) Promotes Increased Proportions of the Highly Permissive Th17-like Cell Profile during HIV Infection" Viruses 14, no. 10: 2218. https://doi.org/10.3390/v14102218