City “Green” Contributions: The Role of Urban Greenspaces as Reservoirs for Biodiversity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Urban Greenspaces
2.3. Sampling Methods
2.4. Statistical Analysis
3. Results
3.1. Species Richness
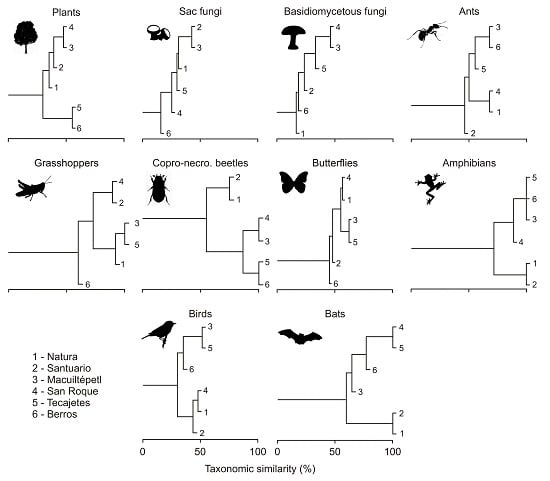
3.2. Composition Similarity
3.3. Greenspace Richness Contribution
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Grimm, N.B.; Faeth, S.H.; Golubiewski, N.E.; Redman, C.L.; Wu, J.; Bai, X.; Brigs, J.M. Global change and the ecology of cities. Science 2008, 319, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, M.R. The urban transformation of the developing world. Science 2008, 319, 761–764. [Google Scholar] [CrossRef] [PubMed]
- United Nations, Department of Economic and Social Affairs, Population Division. World Urbanization Prospects: The 2014 Revision; United Nations: New York, NY, USA, 2015. [Google Scholar]
- Czech, B.; Krausman, P.R. Distribution and causation of species endangerment in the United States. Science 1997, 277, 1116–1117. [Google Scholar] [CrossRef]
- Czech, B.; Krausman, P.R.; Devers, P.K. Economic associations among causes of species endangerment in the United States. Bioscience 2001, 50, 593–601. [Google Scholar] [CrossRef] [Green Version]
- Pickett, S.T.; Cadenasso, M.L.; Grobe, J.M.; Nilon, C.H.; Pouyat, R.B.; Zipperer, W.C.; Costanza, R. Urban ecological systems: Scientific foundations and a decade of progress. J. Environ. Manag. 2011, 92, 331–362. [Google Scholar] [CrossRef] [PubMed]
- McKinney, M.L. Urbanization, biodiversity, and conservation. Bioscience 2002, 52, 883–890. [Google Scholar] [CrossRef]
- McKinney, M.L. Urbanization as a major cause of biotic homogenization. Biol. Conserv. 2006, 127, 247–260. [Google Scholar] [CrossRef]
- McKinney, M.L. Effects of urbanisation on species richness: A review of plants and animals. Urban Ecosyst. 2008, 11, 161–176. [Google Scholar] [CrossRef]
- Radeloff, V.C.; Hammer, R.B.; Stewart, S.I.; Fried, J.S.; Holcomb, S.S.; McKeefry, J.F. The wildland-urban interface in the United States. Ecol. Appl. 2005, 15, 799–805. [Google Scholar] [CrossRef]
- Lawson, D.M.; Lamar, C.K.; Schwartz, M.W. Quantifying plant population persistence in human-dominated landscapes. Conserv. Biol. 2008, 22, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Hahs, A.K.; McDonnell, M.J.; McCarthy, M.A.; Vesk, P.A.; Corlett, R.T.; Norton, B.A.; Clemants, S.E.; Duncan, R.P.; Thompson, K.; Schwarts, M.W.; et al. A global synthesis of plant extinction rates in urban areas. Ecol. Lett. 2009, 12, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- MacGregor-Fors, I.; Morales-Pérez, L.; Schondube, J.E. Does size really matter? Species-area relationships in human settlements. Divers. Distrib. 2011, 17, 112–121. [Google Scholar] [CrossRef]
- Bazzaz, F.A. Plant species diversity in old-field successional ecosystems in southern Illinois. Ecol. Durham 1975, 56, 485–488. [Google Scholar] [CrossRef]
- Hobbs, E.R. Species richness of urban forest patches and implications for urban landscape diversity. Landsc. Ecol. 1988, 1, 141–152. [Google Scholar] [CrossRef]
- Ortega-Álvarez, R.; MacGregor-Fors, I. Living in the big city: Effects of urban land-use on bird community structure, diversity, and composition. Landsc. Urban Plan. 2009, 90, 189–195. [Google Scholar] [CrossRef]
- Nielse, A.B.; van den Bosch, M.; Maruthaveeran, S.; van den Bosch, C.K. Species richness in urban parks and its drivers: A review of empirical evidence. Urban Ecosyst. 2014, 14, 305–327. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Marzluff, J.M.; Bowman, J.M.; Donnelly, R.E. A historical perspective on urban bird research: Trends, terms, and approaches. In Avian Ecology and Conservation in an Urbanizing World; Marzluff, J.M., Bowman, J.M., Donnelly, R.E., Eds.; Springer: Berlin, Germany, 2001; pp. 1–17. [Google Scholar]
- Sattler, T.; Pezzatti, G.B.; Nobis, M.P.; Obrist, M.K.; Roth, T.; Moretti, M. Selection of multiple umbrella species for functional and taxonomic diversity to represent urban biodiversity. Conserv. Biol. 2014, 28, 414–426. [Google Scholar] [CrossRef] [PubMed]
- MacGregor-Fors, I.; Avendaño-Reyes, S.; Bandala, V.M.; Chacón-Zapata, S.; Díaz-Toribio, M.H.; González-García, F.; Lorea-Hernández, F.; Martínez-Gómez, J.; Montes de Oca, E.; Montoya, L.; et al. Multi-taxonomic diversity patterns in a neotropical green city: A rapid biological assessment. Urban Ecosyst. 2015, 18, 633–647. [Google Scholar] [CrossRef]
- Fontana, S.; Sattler, T.; Bontadina, F.; Moretti, M. How to manage the urban green to improve bird diversity and community structure. Landsc. Urban Plan. 2011, 101, 278–285. [Google Scholar] [CrossRef]
- Avila-Campos, J.; Instituto de Ecología, A.C. Personal observation. 2016. [Google Scholar]
- Instituto Nacional de Estadística, Geografía e Informática (INEGI). Censo Nacional de Población y Vivienda 2010; INEGI: Aguascalientes, Mexico, 2010. [Google Scholar]
- Lemoine Rodríguez, R. Cambios en la Cobertura Vegetal de la Ciudad de Xalapa-Enríquez, Veracruz y Zonas Circundantes Entre 1950 y 2010. Master’s Thesis, Universidad Veracruzana, Xalapa, Mexico, 31 October 2012. [Google Scholar]
- Lot, A.; Chiang, F. Manual de Herbario; Consejo Nacional de la Flora de Mexico: Mexico City, Mexico, 1986. [Google Scholar]
- Mueller, G.M.; Bills, G.F.; Foster, S.M. Biodiversity of Fungi: Inventory and Monitoring Methods; Elsevier Academic Press: San Diego, CA, USA, 2004. [Google Scholar]
- Dennis, R.W.G. British Ascomycetes; J. Cramer: Vaduz, Liechtenstein, 1978. [Google Scholar]
- Breitenbach, J.; Kränzlin, F. Fungi of Switzerland. Vol. 1. Ascomycetes; VerlagMykologia: Lucerne, Switzerland, 1984. [Google Scholar]
- O’Dell, T.E.; Lodge, D.J.; Mueller, G.M. Approaches to Sampling Macrofungi. In Biodiversity of Fungi—An Inventory and Monitoring Methods; Elsevier Academic Press: San Diego, CA, USA, 2004. [Google Scholar]
- Largent, D. How to Identify Mushrooms to Genus I—Macroscopic Features; Mad River Press: Eureka, CA, USA, 1973. [Google Scholar]
- Largent, D.; Johnson, D.; Watling, R. How to Identify Mushrooms to Genus III—Microscopic Features; Mad River Press: Eureka, CA, USA, 1977. [Google Scholar]
- Lodge, J.D.; Ammirati, J.F.; O’Dell, T.E.; Mueller, G.M. Collecting and Describing Macrofungi. In Biodiversity of Fungi—An Inventory and Monitoring Methods; Elsevier Academic Press: San Diego, CA, USA, 2004. [Google Scholar]
- Moser, M. Keys to Agarics and Boleti; Roger Phillips: London, UK, 1983. [Google Scholar]
- Pegler, D.N. Agaric flora of the Lesser Antilles; HMSO: London, UK, 1983. [Google Scholar]
- Bessette, A.E.; Bessette, A.R.; Fischer, S.W. Mushrooms of Northeastern North America; Syracuse University Press: Syracuse, NY, USA, 1977. [Google Scholar]
- Horak, E. Röhrlinge und Blätterpilze in Europa; Elsevier Spektrum Akademischer Verlag: Heidelberg, Germany, 2005. [Google Scholar]
- Bestelmeyer, B.T.; Agosti, D.; Alonso, L.E.; Brandao, C.R.F.; Brown, W.L.; Delabie, J.H.C.; Silvestre, R. Field techniques for the study of ground-dwelling ants: An overview, description, and evaluation. In Ants—Standard Methods for Measuring and Monitoring Biodiversity; Agosti, D., Majer, J.D., Alonso, L.E., Shultz, T.R., Eds.; Smithsonian Institution Press: Washington, DC, USA, 2000; pp. 122–144. [Google Scholar]
- MacKay, W.P.; MacKay, E.A. Guide to the species identification of the New World ants. Sociobiology 1989, 16, 3–47. [Google Scholar]
- Bolton, B. Identification Guide to the Ant Genera of the World; Harvard University Press: Cambridge, MA, USA, 1994. [Google Scholar]
- Southwood, T.R.E. Ecological Methods with Particular Reference to the Study of Insect Populations; Springer: London, UK, 1978. [Google Scholar]
- Rivera-Garcia, E. Factores que Determinan la Estructura de las Comunidades de Chapulines (Orthoptera: Acridoidea) en el Desierto Chihuahuense. Ph.D. Thesis, Facultad de Ciencias, UNAM, Mexico City, Mexico, 18 January 2009. [Google Scholar]
- Borror, D.J.; Tripplehorn, C.A.; Johnson, N.F. An Introduction to the Study of Insects; Saunders College Publishing: Philadelphia, PA, USA, 2009. [Google Scholar]
- Morón, M.A.; Terrón, R.A. Entomología Práctica; Instituto de Ecología, A.C.: Mexico City, Mexico, 1988. [Google Scholar]
- Arnett, R.H.; Thomas, M.C. American Beetles, Volume I: Archostemata, Myxophaga, Adephaga, Polyphaga, Staphyliniformia; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Arnett, R.H.; Thomas, M.C.; Skelley, P.E.; Frank, J.H. American Beetles, Volume II: Polyphaga: Scarabaeoidea through Curculionoidea; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Pollard, E.; Yates, T.J. Monitoring Butterflies for Ecology and Conservation; Chapman and Hall: London, UK, 1993. [Google Scholar]
- Glassberg, J.A. Swift Guide to the Butterflies of Mexico and Central America; Sunstreak: Morristown, NJ, USA, 2007. [Google Scholar]
- Hernández Baz, F.; Llorente Bousquets, J.E.; Luis Martínez, A.; Vargas Fernández, I. Las Mariposas de Veracruz—Guía Ilustrada; Consejo Veracruzano de Investigación Ciencia y Desarrollo Tecnológico: Veracruz, Mexico, 2010. [Google Scholar]
- Heyer, R.; Donnelly, M.A.; Foster, M.; Mcdiarmid, R. Measuring and Monitoring Biological Diversity: Standard Methods for Amphibians; Smithsonian Institution: Washington, DC, USA, 2014. [Google Scholar]
- Ralph, C.J.; Droege, S.; Sauer, J.R. Managing and Monitoring Birds Using Point Counts: Standards and Applications; USDA: Albany, NY, USA, 1995. [Google Scholar]
- Ellison, L.E.; Everette, A.L.; Bogan, M.A. Examining patterns of bat activity in Bandelier National Monument, New Mexico, by using walking point transects. Southwest. Nat. 2005, 50, 197–208. [Google Scholar] [CrossRef]
- Fenton, M.B. Reporting: Essential information and analysis. In Bat Echolocation Research: Tools, Techniques and Analysis; Brigham, R.M., Kalko, E.K.V., Jones, G., Parsons, S., Limpens, H.J.A., Eds.; Bat Conservation International: Austin, TX, USA, 2004; pp. 133–140. [Google Scholar]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT Online: Interpolation and Extrapolation (Version 1.0). Available online: https://chao.shinyapps.io/iNEXT (accessed on 27 October 2015).
- Colwell, R.K.; Chao, A.; Gotelli, N.J.; Lin, S.Y.; Mao, C.X.; Chazdon, R.L.; Longino, J.T. Models and estimators linking individual-based and sample-based rarefaction, extrapolation, and comparison of assemblages. J. Plant Ecol. 2012, 5, 3–21. [Google Scholar] [CrossRef]
- MacGregor-Fors, I.; Payton, M. Contrasting diversity values: Statistical inferences based on overlapping confidence intervals. PLoS ONE 2013, 8, e56794. [Google Scholar] [CrossRef]
- Lennon, J.J.; Koleff, P.; Greenwood, J.J.D.; Gaston, K.J. The geographical structure of British bird distri- butions: Diversity, spatial turnover and scale. J. Anim. Ecol. 2001, 70, 966–979. [Google Scholar] [CrossRef]
- Koleff, P.K.; Gaston, K.J.; Lennon, J.J. Measuring beta diversity for presence–absence data. J. Anim. Ecol. 2003, 72, 367–382. [Google Scholar] [CrossRef]
- Gaston, K.J.; Davies, R.G.; Orme, C.D.L.; Olson, V.A.; Thomas, G.H.; Ding, T.S.; Rasmussen, P.C.; Lennon, J.J.; Benneti, P.M.; Owens, I.P.F.; et al. Spatial turnover in the global avifauna. Proc. R. Soc. Lond. B Biol. 2007, 274, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Soykan, C.U.; Brand, L.A.; Ries, L.; Stromberg, J.C.; Hass, C.; Simmons, D.A.; Patterson, W.J.D.; Sabo, J.L. Multitaxonomic diversity patterns along a desert riparian–upland gradient. PLoS ONE 2012, 7, e28235. [Google Scholar] [CrossRef] [PubMed]
- Gavareski, C.A. Relation of park size and vegetation to urban bird populations in Seattle, Washington. Condor 1976, 78, 375–382. [Google Scholar] [CrossRef]
- Puga-Caballero, A.; MacGregor-Fors, I.; Ortega-Alvarez, R. Birds at the urban fringe: Avian community shifts in different peri-urban ecotones of a megacity. Ecol. Res. 2014, 29, 619–628. [Google Scholar] [CrossRef]
- Shwartz, A.; Shirley, S.; Kark, S. How do habitat variability and management regime shape the spatial heterogeneity of birds within a large Mediterranean urban park? Landsc. Urban Plan. 2008, 84, 219–229. [Google Scholar] [CrossRef]
- MacGregor-Fors, I. How to measure the urban-wildland ecotone: Redefining ‘peri-urban’ areas. Ecol. Res. 2010, 25, 883–887. [Google Scholar] [CrossRef]
- Fischer, J.D.; Schneider, S.C.; Ahler, A.A.; Miller, J.R. Categorizing wildlife responses to urbanization and conservation implications of terminology. Conserv. Biol. 2015, 29, 1246–1248. [Google Scholar] [CrossRef] [PubMed]
- Moreno, C.E.; Pineda, E.; Escobar, F.; Sánchez-Rojas, G. Shortcuts for biodiversity evaluation: A review of terminology and recommendations for the use of target groups, bioindicators and surrogates. Int. J. Environ. Heal. 2007, 1, 71–86. [Google Scholar] [CrossRef]
- Arias, H.P.F. Los Árboles de la Zona Urbana y Suburbana de Xalapa. Master’s Thesis, Universidad Veracruzana, Xalapa, Mexico, 1983. [Google Scholar]
- García-Campos, H.M. Las áreas verdes públicas de Xalapa. In Ecología Urbana Aplicada a la Ciudad de Xalapa; López-Moreno, I.R., Ed.; Instituto de Ecología, A.C., MAB UNESCO, H. Ayuntamiento de Xalapa: Xalapa, Mexico, 1993; pp. 99–132. [Google Scholar]
- Ruiz-Montiel, C.; Vázquez-Torres, V.; Martínez-Hernández, M.J.; Murrieta-Pérez, L.; Perez-Hernández, M.S. Árboles y arbustos registrados en el Parque Ecológico Molino de San Roque. Madera Bosques 2014, 20, 143–152. [Google Scholar]
- Martínez-Morales, M.A. Nested species assemblages as a tool to detect sensitivity to forest fragmentation: The case of cloud forest birds. Oikos 2005, 108, 634–642. [Google Scholar] [CrossRef]
- Ganzhorn, J.U.; Eisenbeiß, B. The concept of nested species assemblages and its utility for understanding effects of habitat fragmentation. Basic Appl. Ecol. 2001, 2, 87–99. [Google Scholar] [CrossRef]
- O’Dell, T.E.; Lodge, D.J.; Mueller, G.M. Approaches to sampling macrofungi. In Biodiversity of Fungi: Inventory and Monitoring Methods; Mueller, G.M., Bills, G.F., Foster, M.S., Eds.; Elsevier: San Diego, CA, USA, 2004. [Google Scholar]
- Konijnendijk, C.C. The Forest and the City—The Cultural Landscape of Urban Woodland; Springer: Berlin, Germany, 2008. [Google Scholar]
- Evans, K.L.; Newson, S.E.; Gaston, K.J. Habitat influences on urban avian assemblages. IBIS 2009, 151, 19–39. [Google Scholar] [CrossRef]
- Wettstein, W.; Schmid, B. Conservation of arthropod diversity in montane wetlands: Effect of altitude, habitat quality and habitat fragmentation on butterflies and grasshoppers. J. Appl. Ecol. 1999, 36, 363–373. [Google Scholar] [CrossRef]
- Nufio, C.R.; McClenahan, J.L.; Bowers, M.D. Grasshopper response to reductions in habitat area as mediated by subfamily classification and life history traits. J. Insect Conserv. 2011, 15, 409–419. [Google Scholar] [CrossRef]
- Hostetler, M.; Allen, W.; Meurk, C. Conserving urban biodiversity? Creating green infrastructure is only the first step. Landsc. Urban Plan. 2011, 100, 369–371. [Google Scholar] [CrossRef]
- Beninde, J.I.; Veith, M.; Hochkirch, A. Biodiversity in cities needs space: A meta-analysis of factors determining intra-urban biodiversity variation. Ecol. Lett. 2015, 18, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Ruelas, I.E.; Aguilar Rodríguez, S.H. La avifauna urbana del Parque Ecológico Macuiltépetl en Xalapa, Veracruz, Mexico. Ornitol. Neotrop. 2010, 20, 87–103. [Google Scholar]
- González-García, F.; MacGregor-Fors, I.; Straub, R.; Lobato García, J.A. Birds of a neotropical green city: An up-to-date review of the avifauna of the city of Xalapa with additional unpublished records. Urban Ecosyst. 2014, 17, 991–1012. [Google Scholar] [CrossRef]
- MacGregor-Fors, I.; Ortega-Álvarez, R. Fading from the forest: Bird community shifts related to urban park site-specific and landscape traits. Urban For. Urban Green. 2011, 10, 239–246. [Google Scholar] [CrossRef]
- Ávila-Flores, R.; Fenton, M.B. Use of spatial features by foraging insectivorous bats in a large urban landscape. J. Mammal. 2005, 86, 1193–1204. [Google Scholar] [CrossRef]
- Carpaneto, G.M.; Mazziota, A.; Piattella, E. Changes in food resources and conservation of scarab beetles: From sheep to dog dung in a green urban area of Rome (Coleoptera, Scarabaeoidea). Biol. Conserv. 2005, 123, 547–556. [Google Scholar] [CrossRef]
- Guénard, B.; Cardinal-De Casas, A.; Dunn, R.R. High diversity in an urban habitat: Are some animal assemblages resilient to long-term anthropogenic change? Urban Ecosyst. 2015, 18, 449–463. [Google Scholar] [CrossRef]
- MacGregor-Fors, I.; Ortega-Álvarez, R. Ecología urbana: Experiencias en América Latina. Available online: http://www1.inecol.edu.mx/libro_ecologia_urbana (accessed on 30 June 2016).
- Rosenzweig, M.L. Win-Win Ecology: How the Earth’s Species Can Survive in the Midst of Human Enterprise; Oxford University Press: London, UK, 2003. [Google Scholar]
- McDonnell, M.J. Linking and promoting research and practice in the evolving discipline of urban ecology. J. Urban Ecol. 2015, 1, 1–6. [Google Scholar] [CrossRef]
- Ramírez-Restrepo, L.; Cultid-Medina, C.A.; MacGregor-Fors, I. How many butterflies are there in a city of circa half a million people? Sustainability 2015, 7, 8587–8597. [Google Scholar] [CrossRef]
- McDonnell, M.J.; MacGregor-Fors, I. The ecological future of cities. Science 2016, 352, 936–938. [Google Scholar] [CrossRef] [PubMed]



| Natura | Santuario | Macuiltépetl | San Roque | Tecajetes | Berros | |
|---|---|---|---|---|---|---|
| Plants | 50.3 ± 5.5 | 43.1 ± 3.4 | 36.5 ± 2.9 | 33.1 ± 4.0 | 53.0 ± 5.3 | 19.8 ± 8.2 |
| (AB) | (B) | (C) | (C) | (A) | (D) | |
| Sac fungi | 18.9 ± 3.3 | 17.7 ± 2.9 | 19.1 ± 2.4 | 20.1± 3.0 | 18.6 ± 2.7 | 12.9 ± 7.6 |
| (A) | (A) | (A) | (A) | (A) | (A) | |
| Basidiomycetous fungi | 21.9 ± 1.2 | 40.4 ± 1.7 | 20.2 ± 1.5 | 12.7 ± 2.4 | 5.0 ± 1.8 | 13.9 ± 1.6 |
| (B) | (A) | (B) | (C) | (D) | (C) | |
| Ants | 6.4 ± 1.1 | 5.4 ± 0.8 | 8.2 ± 2.2 | 5.0 ± 1.2 | 6.0 ± 0.0 | 12.1 ± 4 |
| (B) | (B) | (AB) | (B) | (B) | (A) | |
| Grasshoppers | 14.5 ± 1.9 | 12.1 ± 2.0 | 10.6 ± 2.0 | 12.8 ± 8.9 | 3 | 5 |
| (A) | (A) | (A) | (A) | |||
| Copro-necrophagous beetles | 4 | 5 | 4 | 5 | 5 | 3 |
| Butterflies | 30.2 ± 2.4 | 22.3 ± 6.2 | 26.5 ± 6.3 | 22.4 ± 4.3 | 27.9 ± 11.4 | 26.2 ± 12.3 |
| (A) | (AB) | (AB) | (B) | (AB) | (AB) | |
| Amphibians | 7 | 5 | 2 | 1 | 1 | 3 |
| Birds | 18.0 ± 2.9 | 14.5 ± 4.7 | 13.1 ± 2.9 | 14.8 ± 3.9 | 8.8 ± 7.1 | 11.3 ± 2.2 |
| (A) | (AB) | (AB) | (AB) | (AB) | (B) | |
| Bats | 5.6 ± 0.4 | 4.6 ± 1.8 | 6.6 ± 3.8 | 3.7 ± 2.1 | 5.7 ± 0.7 | 6 ± 0 |
| (AB) | (AB) | (AB) | (B) | (AB) | (A) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
MacGregor-Fors, I.; Escobar, F.; Rueda-Hernández, R.; Avendaño-Reyes, S.; Baena, M.L.; Bandala, V.M.; Chacón-Zapata, S.; Guillén-Servent, A.; González-García, F.; Lorea-Hernández, F.; et al. City “Green” Contributions: The Role of Urban Greenspaces as Reservoirs for Biodiversity. Forests 2016, 7, 146. https://doi.org/10.3390/f7070146
MacGregor-Fors I, Escobar F, Rueda-Hernández R, Avendaño-Reyes S, Baena ML, Bandala VM, Chacón-Zapata S, Guillén-Servent A, González-García F, Lorea-Hernández F, et al. City “Green” Contributions: The Role of Urban Greenspaces as Reservoirs for Biodiversity. Forests. 2016; 7(7):146. https://doi.org/10.3390/f7070146
Chicago/Turabian StyleMacGregor-Fors, Ian, Federico Escobar, Rafael Rueda-Hernández, Sergio Avendaño-Reyes, Martha Lucía Baena, Víctor M. Bandala, Santiago Chacón-Zapata, Antonio Guillén-Servent, Fernando González-García, Francisco Lorea-Hernández, and et al. 2016. "City “Green” Contributions: The Role of Urban Greenspaces as Reservoirs for Biodiversity" Forests 7, no. 7: 146. https://doi.org/10.3390/f7070146