Quantifying the Impacts of Systemic Acquired Resistance to Pitch Canker on Monterey Pine Growth Rate and Hyperspectral Reflectance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Trials

2.2. Growth Rate Measurements
2.3. Hyperspectral Reflectance Measurements
2.4. Statistical Analyses
3. Results
3.1. Induction of SAR

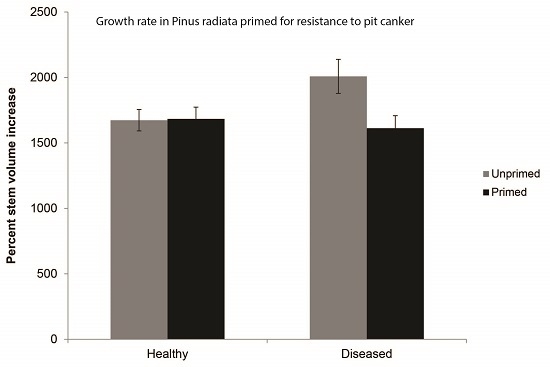
3.2. Treatment Effects on Growth Rate
| Replication 1 | Height (cm) | Diameter (mm) | ||||
|---|---|---|---|---|---|---|
| Mean | Median | Range | Mean | Median | Range | |
| One | 111 | 112 | 46–168 | 24 | 24 | 10–37 |
| Two | 173 | 178 | 60–280 | 37 | 37 | 10–61 |
| Growth Interval 1 | Factor 2 | DF 3 | F-Value | P (>F) 4 |
|---|---|---|---|---|
| Three months | Priming (P) | 1, 312 | 0.50 | 0.6077 |
| Three months | Disease (D) | 1, 312 | 0.38 | 0.6485 |
| Three months | P*D | 1, 312 | 8.45 | 0.0039 |
| Six months | Priming (P) | 1, 314 | 0.15 | 0.6975 |
| Six months | Disease (D) | 1, 314 | 0.71 | 0.3999 |
| Six months | P*D | 1, 314 | 5.93 | 0.0155 |
| Nine months | Priming (P) | 1, 314 | 1.44 | 0.2306 |
| Nine months | Disease (D) | 1, 314 | 2.08 | 0.1503 |
| Nine months | P*D | 1, 314 | 6.29 | 0.0126 |
| Twelve months | Priming (P) | 1, 315 | 3.71 | 0.0551 |
| Twelve months | Disease (D) | 1, 315 | 1.34 | 0.2477 |
| Twelve months | P*D | 1, 315 | 3.60 | 0.0586 |

3.3. Spectral Measurements


4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jones and Stokes Associates. Final. Monterey Pine Forest Ecological Assessment: Historical Distribution, Ecology, and Current Status of Monterey Pine. Available online: http://www.co.monterey.ca.us/planning/major/Pebble%20Beach%20Company/Pebble_Beach_DEIR_Nov_2011/Pebble_Beach_DEIR_Admin_Records_Nov_2011/Jones_Stokes/JS_1994_MPF_Eco_Assess.pdf (accessed on 4 January 2016).
- Rogers, D.L.; Vargas Hernández, J.J.; Matheson, A.C.; Guerra Santos, J.J. The Mexican Island Populations of Pinus Radiata: An International Expedition and Ongoing Collaboration for Genetic Conservation. Available online: http://www.fao.org/docrep/005/y4341e/y4341e07.htm#P947_91075/ (accessed on 4 January 2016).
- Rogers, D.L. In situ genetic conservation of a naturally restricted and commercially widespread species, Pinus radiata. For. Ecol. Manag. 2004, 197, 311–322. [Google Scholar] [CrossRef]
- Hepting, G.H.; Roth, E.R. Pitch canker, a new disease of some southern pines. J. For. 1946, 44, 724–744. [Google Scholar]
- McCain, A.H.; Koehler, C.S.; Tjosvold, S.A. Pitch canker threatens California pines. Calif. Agric. 1987, 41, 22–23. [Google Scholar]
- Gordon, T.R.; Storer, A.J.; Wood, D.L. The pitch canker epidemic in California. Plant Dis. 2001, 85, 1128–1139. [Google Scholar] [CrossRef]
- Walters, D.; Walsh, D.; Newton, A.; Lyon, G. Induced resistance for plant disease control: Maximizing the efficacy of resistance elicitors. Phytopathology 2005, 95, 1368–1373. [Google Scholar] [CrossRef] [PubMed]
- Durrant, W.E.; Dong, X. Systemic acquired resistance. Annu. Rev. Phytopathol. 2004, 42, 185–209. [Google Scholar] [CrossRef] [PubMed]
- Bonello, P.; Gordon, T.R.; Storer, A.J. Systemic induced resistance in Monterey pine. For. Pathol. 2001, 31, 99–106. [Google Scholar] [CrossRef]
- Gordon, T.R.; Kirkpatrick, S.C.; Aegerter, B.J.; Fisher, A.J.; Storer, A.J.; Wood, D.L. Evidence for the occurrence of induced resistance to pitch canker, caused by Gibberella circinata (anamorph Fusarium circinatum), in populations of Pinus radiata. For. Pathol. 2011, 41, 227–232. [Google Scholar] [CrossRef]
- Storer, A.J.; Wood, D.L.; Gordon, T.R. The epidemiology of pitch canker in California. For. Sci. 2002, 48, 694–700. [Google Scholar]
- Heil, M.; Hilpert, A.; Kaiser, W.; Linsenmair, K.E. Reduced growth and seed set following chemical induction of pathogen defence: Does systemic acquired resistance (SAR) incur allocation costs? J. Ecol. 2000, 88, 645–654. [Google Scholar] [CrossRef]
- Dietrich, R.; Ploss, K.; Heil, M. Growth responses and fitness costs after induction of pathogen resistance depend on environmental conditions. Plant Cell Environ. 2005, 28, 211–222. [Google Scholar] [CrossRef]
- Smedegaard-Petersen, V.; Stølen, O. Effect of energy-requiring defense reactions on yield and grain quality in a powdery mildew-resistant barley cultivar. Phytopathology 1981, 71, 396–399. [Google Scholar] [CrossRef]
- Tuzun, S.; Kuc, J. A modified technique for inducing systemic resistance to blue mold and increasing growth in tobacco. Phytopathology 1985, 75, 1127–1129. [Google Scholar] [CrossRef]
- Van Hulten, M.; Pelser, M.; van Loon, L.C.; Pieterse, C.M.J.; Ton, J. Costs and benefits of priming for defense in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 5602–5607. [Google Scholar] [CrossRef] [PubMed]
- Aegerter, B.J.; Gordon, T.R. Rates of pitch canker induced seedling mortality among Pinus radiata families varying in levels of genetic resistance to Gibberella circinata (anamorph Fusarium circinatum). For. Ecol. Manag. 2006, 235, 14–17. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A.; Bullock, S. The Fusarium Lab Manual; Blackwell: Ames, IA, USA, 2006. [Google Scholar]
- Knipling, E.B. Physical and physiological basis for the reflectance of visible and near-infrared radiation from vegetation. Remote Sens. Environ. 1970, 1, 155–159. [Google Scholar] [CrossRef]
- Reynolds, G.J.; Windels, C.E.; MacRae, I.V.; Laguette, S. Remote sensing for assessing Rhizoctonia crown and root rot severity in sugar beet. Plant Dis. 2012, 96, 497–505. [Google Scholar] [CrossRef]
- Seelig, H.D.; Hoehn, A.; Stodieck, L.S.; Klaus, D.M.; Adams, W.W., III; Emery, W.K. Relations of remote sensing leaf water indices to leaf water thickness in cowpea, bean, and sugarbeet plants. Remote Sens. Environ. 2008, 112, 445–455. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring vegetation systems in the Great Plains with ERTS. In Third Earth Resources Technology Satellite-1 Symposium; Stanley, C.F., Enrico, P.M., Margaret, A.B., Eds.; NASA: Washington, DC, USA, 1973; pp. 309–317. [Google Scholar]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of soil-adjusted vegetation indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Heil, M.; Baldwin, I.T. Fitness costs of induced resistance: Emerging experimental support for a slippery concept. Trends Plant Sci. 2002, 7, 61–67. [Google Scholar] [CrossRef]
- Takatsuji, H. Development of disease-resistant rice using regulatory components of induced resistance. Front. Plant Sci. 2014, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bonello, P.; Gordon, T.R.; Herms, D.A.; Wood, D.L.; Erbilgin, N. Nature and ecological implications of pathogen-induced systemic resistance in conifers: A novel hypothesis. Physiol. Mol. Plant Pathol. 2006, 68, 95–104. [Google Scholar] [CrossRef]
- Jordan, C.F. Derivation of leaf area index from quality of light on the forest floor. Ecology 1969, 50, 663–666. [Google Scholar] [CrossRef]
- Merton, R.N.; Harvey, L.E. Analysis of seasonal changes in Jasper Ridge vegetation biochemistry and biophysiology using multitemporal hyperspectral data. In Proceedings of the American Society for Photogrammetry and Remote Sensing (ASPRS) Annual Conference, Seattle, WA, USA, 7–10 April 1997.
- Blodgett, J.T.; Eyles, A.; Bonello, P. Organ-dependant induction of systemic resistance and systemic susceptibility in Pinus nigra inoculated with Sphaeropsis sapinea and Diplodia scrobiculata. Tree Physiol. 2007, 27, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Reglinski, T.; Taylor, J.T.; Dick, M.A. Chitosan induces resistance to pitch canker in Pinus radiata. N. Z. J. For. Sci. 2004, 34, 49–58. [Google Scholar]
- Fitza, K.N.E.; Payn, K.G.; Steenkamp, E.T.; Myburg, A.A.; Naidoo, S. Chitosan application improves resistance to Fusarium circinatum in Pinus radiata. S. Afr. J. Bot. 2013, 85, 70–78. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reynolds, G.J.; Gordon, T.R.; McRoberts, N. Quantifying the Impacts of Systemic Acquired Resistance to Pitch Canker on Monterey Pine Growth Rate and Hyperspectral Reflectance. Forests 2016, 7, 20. https://doi.org/10.3390/f7010020
Reynolds GJ, Gordon TR, McRoberts N. Quantifying the Impacts of Systemic Acquired Resistance to Pitch Canker on Monterey Pine Growth Rate and Hyperspectral Reflectance. Forests. 2016; 7(1):20. https://doi.org/10.3390/f7010020
Chicago/Turabian StyleReynolds, Gregory J., Thomas R. Gordon, and Neil McRoberts. 2016. "Quantifying the Impacts of Systemic Acquired Resistance to Pitch Canker on Monterey Pine Growth Rate and Hyperspectral Reflectance" Forests 7, no. 1: 20. https://doi.org/10.3390/f7010020