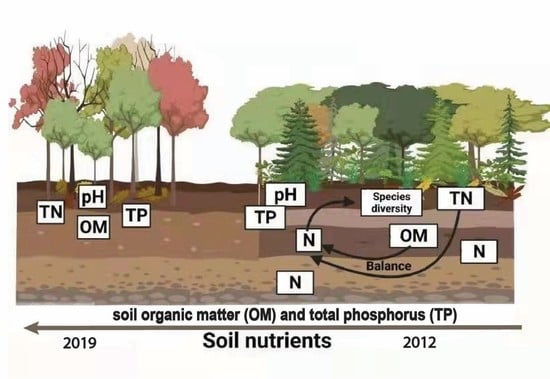
Plant Diversity and Soil Nutrients in a Tropical Coastal Secondary Forest: Association Ordination and Sampling Year Differences
Abstract
:1. Introduction
2. Material and Methods
2.1. Site Conditions
2.2. Data Collection
2.3. Soil Analysis
2.4. Data Analysis
3. Results
3.1. Patterns of Soil Nutrients and Effects on Plant Diversity in Two Different Years
3.2. Patterns of Dominant Species and Effects of Soil Nutrients on Dominant Species (Based on Their Basal Area and Abundance)
3.3. Variation in Soil Nutrients in Two Different Years
4. Discussion
4.1. Patterns of Soil Nutrients and Their Effects on Species Diversity in Two Different Sampling Years
4.2. Soil Properties Differences in Two Different Years
4.3. Plant Species Diversity and Dominant Species Role in Two Different Sampling Years and Their Relationship to Soil Nutrients (2012 and 2019)
5. Significance for Conservation
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fujii, K.; Shibata, M.; Kitajima, K.; Ichie, T.; Kitayama, K.; Turner, B.L. Plant–soil interactions maintain biodiversity and functions of tropical forest ecosystems. Ecol. Res. 2018, 33, 149–160. [Google Scholar] [CrossRef]
- Liu, X.; Tan, N.; Zhou, G.; Zhang, D.; Zhang, Q.; Liu, S.; Chu, G.; Liu, J. Plant diversity and species turnover co-regulate soil nitrogen and phosphorus availability in Dinghushan forests, southern China. Plant Soil 2021, 464, 257–272. [Google Scholar] [CrossRef]
- Trindade, C.R.T.; Landeiro, V.L.; Schneck, F. Macrophyte functional groups elucidate the relative role of environmental and spatial factors on species richness and assemblage structure. Hydrobiologia 2018, 823 Pt 2, 217–230. [Google Scholar] [CrossRef]
- Dong, S.; Sha, W.; Su, X.; Zhang, Y.; LI, S.; Gao, X.; Liu, S.; Shi, J.; Liu, Q.; Hao, Y. The impacts of geographic, soil and climatic factors on plant diversity, biomass and their relationships of the alpine dry ecosystems: Cases from the Aerjin Mountain Nature Reserve, China. Ecol. Eng. 2019, 127, 170–177. [Google Scholar] [CrossRef]
- Deyn, D.E.; Raaijmakers, G.B.; Van, C.E.; Der Putten, W.H. Plant community development is affected by nutrients and soil biota. J. Ecol. 2004, 92, 824–834. [Google Scholar] [CrossRef]
- Perroni-Ventura, Y.; Montaña, C.; García-Oliva, F. Relationship between soil nutrient availability and plant species richness in a tropical semi-arid environment. J. Veg. Sci. 2006, 17, 719–728. [Google Scholar] [CrossRef]
- Bulenga, G.B.; Maliondo, S.M.S.; Katani, J.Z. Relationships between tree species diversity with soil chemical properties in semi-dry Miombo Woodland ecosystems. Tanzan. J. For. Nat. Conserv. 2021, 90, 1–17. [Google Scholar]
- Odum, E.P. The Strategy of Ecosystem Development. Science 1969, 164, 262–270. [Google Scholar] [CrossRef] [Green Version]
- Turner, B.L.; Brenes-Arguedas, T.; Condit, R. Pervasive phosphorus limitation of tree species but not communities in tropical forests. Nature 2018, 555, 367–370. [Google Scholar] [CrossRef]
- Zalamea, P.; Turner, B.L.; Winter, K.; Jones, F.A.; Sarmiento, C.; Dalling, J.W. Seedling growth responses to phosphorus reflect adult distribution patterns of tropical trees. New Phytol. 2016, 212, 400–408. [Google Scholar] [CrossRef]
- Jakovac, C.C.; Bongers, F.; Kuyper, T.W.; Mesquita, R.C.G.; Pena-Claros, M. Land use as a filter for species composition in Amazonian secondary forests. J. Veg. Sci. 2016, 27, 1104–1116. [Google Scholar] [CrossRef] [Green Version]
- Grime, J.P. Plant Strategies and Vegetation Processes, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 1979; ISBN 9780471996927. [Google Scholar]
- Long, C.; Yang, X.; Long, W.; Li, D.; Zhou, W.; Zhang, H. Soil nutrients influence plant community assembly in two tropical coastal secondary forests. Trop. Conserv. Sci. 2018, 11, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Gaston, K.J. Common ecology. Bioscience 2011, 61, 354–362. [Google Scholar] [CrossRef]
- Neri, A.V.; Schaefer, C.E.G.R.; Silva, A.F.; Souza, A.L.; Ferreira-Junior, W.G.; Meira-Neto, J.A.A. The influence of soils on the floristic composition and community structure of an area of Brazilian cerrado vegetation. Edinb. J. Bot. 2012, 69, 1–27. [Google Scholar] [CrossRef]
- Janssen, F.; Peters, A.; Tallowin, J.R.B.; Bakker, J.P.; Bekker, R.M.; Fillat, F.; Oomes, M.J.M. Relationship between soil chemical factors and grassland diversity. Plant Soil 1998, 202, 69–78. [Google Scholar] [CrossRef]
- Kumar, J.I.; Kumar, R.N.; Bhoi, R.K.; Sajish, P.R. Tree species diversity and soil nutrient status in three sites of tropical dry deciduous forest of western India. Trop. Ecol. 2010, 51, 273–279. [Google Scholar]
- Nadeau, M.B.; Sullivan, T.P. Relationships between plant biodiversity and soil fertility in a mature tropical forest, Costa Rica. Int. J. For. Res. 2015, 2015, 732946. [Google Scholar] [CrossRef]
- Cárate-Tandalla, D.; Camenzind, T.; Leuschner, C.; Homeier, J. Contrasting species responses to continued nitrogen and phosphorus addition in tropical montane forests tree seedlings. Biotropica 2018, 50, 234–245. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Q.; Liu, X.; Xu, Z.; Dai, Y.; Liu, Y.; Chen, Y. Variation patterns of plant composition/diversity in Dacrydium pectinatum communities and their driving factors in a biodiversity hotspot on Hainan Island, China. Glob. Ecol. Conserv. 2020, 22, e01034. [Google Scholar] [CrossRef]
- John, R.; Dalling, J.W.; Harms, K.E.; Yavitt, J.B.; Stallard, R.F.; Mirabello, M.; Hubbell, S.P.; Valencia, R.; Navarrete, H.; Vallejo, M.; et al. Soil nutrients influence spatial distributions of tropical tree species. Proc. Natl. Acad. Sci. USA 2007, 104, 864–869. [Google Scholar] [CrossRef] [Green Version]
- Fayolle, A.; Engelbrecht, B.; Freycon, V.; Mortier, F.; Swaine, M.; Rejou Mechain, M.; Doucet, J.-L.; Fauvet, N.; Cornu, G.; Gourlet-Fleury, S. Geological substrates shape tree species and trait distributions in African moist forests. PLoS ONE 2012, 7, e42381. [Google Scholar] [CrossRef]
- Figueiredo, F.O.; Zuquim, G.; Tuomisto, H.; Moulatlet, G.M.; Balslev, H.; Costa, F.R. Beyond climate control on species range: The importance of soil data to predict distribution of Amazonian plant species. J. Biogeogr. 2018, 45, 190–200. [Google Scholar] [CrossRef]
- Werden, L.K.; Becknell, J.M.; Powers, J.S. Edaphic factors, successional status and functional traits drive habitat associations of trees in naturally regenerating tropical dry forests. Funct. Ecol. 2018, 32, 2766–2776. [Google Scholar] [CrossRef]
- Augusto, L.; Achat, D.L.; Jonard, M.; Vidal, D.; Ringeval, B. Soil parent material—A major driver of plant nutrient limitations in terrestrial ecosystems. Glob. Change Biol. 2017, 23, 3808–3824. [Google Scholar] [CrossRef] [PubMed]
- Medvigy, D.; Wang, G.; Zhu, Q.; Riley, W.J.; Trierweiler, A.M.; Waring, B.G.; Xu, X.; Powers, J.S. Observed variation in soil properties can drive large variation in modelled forest functioning and composition during tropical forest secondary succession. New Phytol. 2019, 223, 1820–1833. [Google Scholar] [CrossRef]
- Situ, S.J. Study on the Historical Land Exploitation of Hainan Island; Hainan Press: Haikou, China, 1987. [Google Scholar]
- Waring, B.G.; Becknell, J.M.; Powers, J.S. Nitrogen, phosphorus, and cation use efficiency in stands of regenerating tropical dry forest. Oecologia 2015, 178, 887–897. [Google Scholar] [CrossRef]
- Huang, J.; Wang, C.; Qi, L.; Zhang, X.; Tang, G.; Li, L.; Guo, J.; Jia, Y.; Dou, X.; Lu, M. Phosphorus is more effective than nitrogen in restoring plant communities of heavy metals polluted soils. Environ. Pollut. 2020, 266, 115259. [Google Scholar] [CrossRef]
- Long, W.; Yang, X.; Li, D. Patterns of species diversity and soil nutrients along a chrono sequence of vegetation recovery in Hainan Island, South China. Ecol. Res. 2012, 27, 561–568. [Google Scholar] [CrossRef]
- Wu, Z.Y. Vegetation in China; Science Press: Beijing, China, 1995. [Google Scholar]
- Jobba’gy, E.G.; Jackson, R.B. The distribution of soil nutrients with depth: Global patterns and the imprint of plants. Biogeochemistry 2001, 53, 51–77. [Google Scholar] [CrossRef]
- Anderson, J.M.; Ingram, J.S.I. Tropical Soil Biology and Fertility: A Handbook of Methods; C.A.B. International: Aberystwyth, UK, 1989; Volume 157, p. 265. [Google Scholar]
- Jung, S.-H. Stratified Fisher’s exact test and its sample size calculation. Biom. J. 2014, 56, 129–140. [Google Scholar] [CrossRef] [Green Version]
- R Core Development Team (Ed.) R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: www.R-project.org/ (accessed on 10 February 2015).
- Agbu, P.A.; Olson, K.R. Spatial variability of soil properties in selected Illinois Mollisols. Soil Sci. 1990, 150, 777–786. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Wu, J.; Shen, Z.; Zhang, Y.; Xu, X.; Fan, Y.; Zhao, Y.; Yan, W. Root biomass distribution in alpine ecosystems of the northern Tibetan Plateau. Environ. Earth Sci. 2011, 64, 1911–1919. [Google Scholar] [CrossRef]
- Chen, C.R.; Condron, L.M.; Davis, M.R.; Sherlock, R.R. Effects of afforestation on phosphorus dynamics and biological properties in a New Zealand grassland soil. Plant Soil 2000, 220, 151–163. [Google Scholar] [CrossRef]
- Kooyman, R.M.; Laffan, S.W.; Westoby, M. The incidence of low phosphorus soils in Australia. Plant Soil 2017, 412, 143–150. [Google Scholar] [CrossRef]
- Smithsonian Tropical Research Institute. Diverse Tropical Forests Grow Fast Despite Widespread Phosphorus Limitation. ScienceDaily. 7 March 2018. Available online: www.sciencedaily.com/releases/2018/03/180307153944.htm (accessed on 7 March 2018).
- Bohn, H.; Mcneal, B.; O’Connor, G. Soil Chemistry, 3rd ed.; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen–phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Zang, R.; Letcher, S.G.; Ding, Y.; Huang, Y.; Lu, X.; Huang, J.; Liu, W.; Zhang, Z. Associations between plant composition/diversity and the abiotic environment across six vegetation types in a biodiversity hotspot of Hainan Island, China. Plant Soil 2016, 403, 21–35. [Google Scholar] [CrossRef]
- ter Steege, H.; Pitman, N.C.A.; Killeen, T.J.; Laurance, W.F.; Peres, C.A.; Guevara, J.E.; Salomão, R.P.; Castilho, C.V.; Amaral, I.L.; de Almeida Matos, F.D.; et al. Estimating the global conservation status of more than 15,000 Amazonian tree species. Sci. Adv. 2015, 1. [Google Scholar] [CrossRef] [Green Version]
- Kettle, C.J.; Koh, L.P. (Eds.) Global Forest Fragmentation; CABI: Wallingford, UK, 2014; ISBN 9781780642031. [Google Scholar]
- Hedin, L.O.; Brookshire, E.N.J.; Menge, D.N.L.; Barron, A.R. The Nitrogen Paradox in Tropical Forest Ecosystems. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 613–635. [Google Scholar] [CrossRef] [Green Version]
- LeBauer, D.S.; Treseder, K.K. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 2008, 89, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Avolio, M.L.; Forrestel, E.J.; Chang, C.C.; La Pierre, K.J.; Burghardt, K.T.; Smith, M.D. Demystifying dominant species. New Phytol. 2019, 223, 1106–1126. [Google Scholar] [CrossRef]
- Chen, J.; Qu, M.; Zhang, J.; Xie, E.; Huang, B.; Zhao, Y. Soil fertility quality assessment based on geographically weighted principal component analysis (GWPCA) in large-scale areas. CATENA 2021, 201, 105197. [Google Scholar] [CrossRef]
- Hernández-Vargas, G.; Sánchez-Velásquez, L.R.; López-Acosta, J.C.; Noa-Carrazana, J.C.; Perroni, Y. Relationship between soil properties and leaf functional traits in early secondary succession of tropical montane cloud forest. Ecol. Res. 2019, 34, 213–224. [Google Scholar] [CrossRef]
- Whittaker, R.J.; Bush, M.B.; Richards, K. Plant Recolonization and Vegetation Succession on the Krakatau Islands, Indonesia. Ecol. Monogr. 1989, 59, 59–123. [Google Scholar] [CrossRef]
- Schmidt, M.; Veldkamp, E.; Corre, M.D. Tree species diversity effects on productivity, soil nutrient availability and nutrient response efficiency in a temperate deciduous forest. For. Ecol. Manag. 2015, 338, 114–123. [Google Scholar] [CrossRef]
- Chala, D.; Brochmann, C.; Psomas, A.; Ehrich, D.; Gizaw, A.; Masao, C.A.; Zimmermann, N.E. Good-bye to tropical alpine plant giants under warmer climates? Loss of range and genetic diversity in Lobelia rhynchopetalum. Ecol. Evol. 2016, 6, 8931–8941. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Rivera, H.; Currie, D.J. Contemporaneous climate directly controls broad-scale patterns of woody plant diversity: A test by a natural experiment over 14,000 years. Glob. Ecol. Biogeogr. 2015, 24, 97–106. [Google Scholar] [CrossRef]
- Kreft, H.; Jetz, W. Global patterns and determinants of vascular plant diversity. Proc. Natl. Acad. Sci. USA 2007, 104, 5925–5930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitfeld, T.J.; Lasky, J.R.; Damas, K.; Sosanika, G.; Molem, K.; Montgomery, R.A. Species richness, forest structure, and functional diversity during succession in the New Guinea lowlands. Biotropica 2014, 46, 538–548. [Google Scholar] [CrossRef]
- Lu, X.; Zang, R.; Ding, Y.; Huang, J. Partitioning the functional variation of tree seedlings during secondary succession in a tropical lowland rainforest. Ecosphere 2018, 9, e02305. [Google Scholar] [CrossRef]
- Li, C.; Zhao, L.; Sun, P.; Zhao, F.; Kang, D.; Yang, G.; Han, X.; Feng, Y.; Ren, G. Deep Soil C, N, and P Stocks and Stoichiometry in Response to Land Use Patterns in the Loess Hilly Region of China. PLoS ONE 2016, 11, e0159075. [Google Scholar] [CrossRef]



| Parameters | Two Years’ Difference | Wilcoxon’s Rank Test | ||
|---|---|---|---|---|
| 2012 | 2019 | W | p-Value | |
| Shannon–Wiener index | 3.21 ± 0.02 | 2.84 ± 0.09 | 6100.0 | 0.01 |
| Simpson index | 0.94 ± 0.01 | 0.88 ± 0.02 | 5907.0 | 0.01 |
| Species richness | 45.07 ± 0.8 | 35.92 ± 1.56 | 6462.5 | 0.00 |
| Pielou’s evenness | 0.85 ± 0.01 | 0.85 ± 0.01 | 4608.0 | 0.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaseen, M.; Fan, G.; Zhou, X.; Long, W.; Feng, G. Plant Diversity and Soil Nutrients in a Tropical Coastal Secondary Forest: Association Ordination and Sampling Year Differences. Forests 2022, 13, 376. https://doi.org/10.3390/f13030376
Yaseen M, Fan G, Zhou X, Long W, Feng G. Plant Diversity and Soil Nutrients in a Tropical Coastal Secondary Forest: Association Ordination and Sampling Year Differences. Forests. 2022; 13(3):376. https://doi.org/10.3390/f13030376
Chicago/Turabian StyleYaseen, Muhammad, Gaopan Fan, Xingcui Zhou, Wenxing Long, and Guang Feng. 2022. "Plant Diversity and Soil Nutrients in a Tropical Coastal Secondary Forest: Association Ordination and Sampling Year Differences" Forests 13, no. 3: 376. https://doi.org/10.3390/f13030376