Stable Coexistence in a Field-Calibrated Individual-Based Model of Mangrove Forest Dynamics Caused by Inter-Specific Crown Plasticity
Abstract
:1. Introduction
1.1. Theories of Plant Competition
1.2. Recent Developments of Individual-Based Modeling
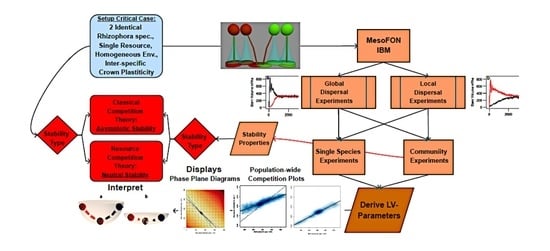
1.3. Objectives of This Study
2. Materials and Methods
2.1. MesoFON—An Individual-Based Model of Mangrove Forest Dynamics
2.1.1. Tree Recruitment
2.1.2. Tree Growth
2.1.3. Growth Reduction Due to Competition
2.1.4. (Inter-Specific) Crown Plasticity
2.1.5. Tree Death
2.2. Conducting MesoFON Simulation Experiments
2.3. Parameterization of the Lotka–Volterra (LV) Model from Simulation Experiments
- r1 and r2 denote the intrinsic volume-specific growth rates for species 1 and 2 in m3 ha−1 yr−1, respectively;
- V1 and V2 represent the respective stem volumes in m3 ha−1;
- K1 and K2 are the respective carrying capacities, also given in m3 ha−1;
- α1←2 is the inter-specific competition coefficient of species 2 on species 1;
- α2←1 is the inter-specific competition coefficient of species 1 on species 2;
- α11 and α22 are the intra-specific competition coefficients of species 1 and 2, respectively.
3. Results
3.1. Community Dynamics in the Individual-Based Model
3.2. Lotka–Volterra Parameters from Single-Species Experiments
3.3. Lotka–Volterra Parameters from Community Experiments with Global Dispersal
3.4. Lotka–Volterra Parameters from Community Experiments with Localized Dispersal
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. The Three Theories of Plant Competition
Appendix A.1. Classical Competition Theory: The Lotka–Volterra (LV) Model
Appendix A.2. Resource Limitation Theory
Appendix A.2.1. The Resource Ratio Hypothesis
Appendix A.2.2. The Resource Co-Limitation Theory
Appendix B

Appendix C

References
- Grimm, V.; Railsback, S.F. Individual-Based Modeling and Ecology; Princeton University Press: Princeton, NJ, USA, 2005; ISBN 9780691096667. [Google Scholar]
- Pacala, S.W.; Levin, S.A. Biologically generated spatial pattern and the coexistence of competing species. In Spatial Ecology: The Role of Space in Population Dynamics and Interspecific Interactions; Princeton University Press: Princeton, NJ, USA, 1997; pp. 204–232. [Google Scholar]
- Law, R.; Dieckmann, U.; Metz, J.A.J. Introduction. In The Geometry of Ecological Interactions: Simplifying Spatial Complexity; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2000; pp. 1–6. ISBN 9780521642941. [Google Scholar]
- Neuhauser, C.; Pacala, S.W. An Explicitly Spatial Version of the Lotka-Volterra Model with Interspecific Competition. Ann. Appl. Probab. 1999, 9, 1226–1259. [Google Scholar]
- Pacala, S.W.; Deutschman, D.H. Details that matter: The spatial distribution of individual trees maintains forest ecosystem function. Oikos 1995, 74, 357. [Google Scholar] [CrossRef]
- Lotka, A.J. Elements of Physical Biology; Williams & Wilkins Company: Baltimore, MD, USA, 1925. [Google Scholar]
- Volterra, V. Variazioni e Fluttuazioni del Numero d’individui in Specie Animali Conviventi, Memoria del Socio Vito Volterra; Società Anonima Tipografica Leonardo da Vinci: Città di Castello, Italy, 1926. [Google Scholar]
- Tilman, D. Resource Competition and Community Structure; Princeton University Press: Princeton, NJ, USA, 1982; ISBN 9780691083025. [Google Scholar]
- Elser, J.J.; Bracken, M.E.S.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harpole, W.S.; Ngai, J.T.; Cleland, E.E.; Seabloom, E.W.; Borer, E.T.; Bracken, M.E.S.; Elser, J.J.; Gruner, D.S.; Hillebrand, H.; Shurin, J.B.; et al. Nutrient co-limitation of primary producer communities. Ecol. Lett. 2011, 14, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, K.W.; Kerimoglu, O. Autotrophic stoichiometry emerging from optimality and variable co-limitation. Front. Ecol. Evol. 2016, 4, 131. [Google Scholar] [CrossRef] [Green Version]
- Adams, T.; Ackland, G.; Marion, G.; Edwards, C. Effects of local interaction and dispersal on the dynamics of size-structured populations. Ecol. Model. 2011, 222, 1414–1422. [Google Scholar] [CrossRef] [Green Version]
- Murrell, D.J. When does local spatial structure hinder competitive coexistence and reverse competitive hierarchies? Ecology 2010, 91, 1605–1616. [Google Scholar] [CrossRef] [Green Version]
- Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Evol. Syst. 2000, 31, 343–366. [Google Scholar] [CrossRef] [Green Version]
- Stoll, P.; Prati, D. Intraspecific aggregation alters competitive interactions in experimental plant communities. Ecology 2001, 82, 319–327. [Google Scholar] [CrossRef]
- Levine, J.M.; Murrell, D.J. The community-level consequences of seed dispersal patterns. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 549–574. [Google Scholar] [CrossRef] [Green Version]
- Barot, S. Mechanisms promoting plant coexistence: Can all the proposed processes be reconciled? Oikos 2004, 106, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Strigul, N.; Pristinski, D.; Purves, D.W.; Dushoff, J.; Pacala, S.W. Scaling from trees to forests: Tractable macroscopic equations for forest dynamics. Ecol. Monogr. 2008, 78, 523–545. [Google Scholar] [CrossRef] [Green Version]
- Pacala, S.W.; Canham, C.D.; Saponara, J.; Silander, J.A., Jr.; Kobe, R.K.; Ribbens, E. Forest models defined by field measurements: Estimation, error analysis and dynamics. Ecol. Monogr. 1996, 66, 1–43. [Google Scholar] [CrossRef]
- Grueters, U.; Seltmann, T.; Schmidt, H.; Horn, H.; Pranchai, A.; Vovides, A.G.; Peters, R.; Vogt, J.; Dahdouh-Guebas, F.; Berger, U. The mangrove forest dynamics model mesoFON. Ecol. Model. 2014, 291, 28–41. [Google Scholar] [CrossRef]
- Berger, U.; Hildenbrandt, H. A new approach to spatially explicit modelling of forest dynamics: Spacing, ageing and neighbourhood competition of mangrove trees. Ecol. Model. 2000, 132, 287–302. [Google Scholar] [CrossRef]
- Pretzsch, H. Forest Dynamics, Growth and Yield from Measurement to Model; Springer: Berlin, Germany; London, UK, 2009; ISBN 9783540883074. [Google Scholar]
- Grueters, U.; Ibrahim, M.R.; Satyanarayana, B.; Dahdouh-Guebas, F. Individual-based modeling of mangrove forest growth: MesoFON—Recent calibration and future direction. Estuar. Coast. Shelf Sci. 2019, 227, 106302. [Google Scholar] [CrossRef] [Green Version]
- Dahdouh-Guebas, F.; Hugé, J.; Abuchahla, G.M.O.; Cannicci, S.; Jayatissa, L.P.; Kairo, J.G.; Kodikara Arachchilage, S.; Koedam, N.; Mafaziya Nijamdeen, T.W.G.F.; Mukherjee, N.; et al. Reconciling nature, people and policy in the mangrove social-ecological system through the adaptive cycle heuristic. Estuar. Coast. Shelf Sci. 2021, 248, 106942. [Google Scholar] [CrossRef]
- Yin, R.K. Case Study Research: Design and Methods, 4th ed.; Sage: Los Angeles, CA, USA, 2009; ISBN 9781412960991. [Google Scholar]
- Flyvbjerg, B. Chapter 17: Case study. In The SAGE Handbook of Qualitative Research, 4th ed.; Denzin, N.K., Lincoln, Y.S., Eds.; Sage: New York, NY, USA, 2011; pp. 301–316. ISBN 9781412974172. [Google Scholar]
- Ridder, H.-G. Case Study Research: Approaches, Methods, Contribution to Theory; Rainer Hampp: München, Mering, 2016; ISBN 9783957101754. [Google Scholar]
- Grover, J.P. Resource Competition, 1st ed.; Chapman & Hall: London, UK; New York, NY, USA, 1997. [Google Scholar]
- Pretzsch, H.; Forrester, D.I.; Bauhus, J. Mixed-Species Forests: Ecology and Management; Springer: Berlin, Germany, 2017; ISBN 978-3662545515. [Google Scholar]
- Sengupta, R.; Middleton, B.; Yan, C.; Zuro, M.; Hartman, H. Landscape characteristics of Rhizophora mangle forests and propagule deposition in coastal environments of Florida (USA). Landsc. Ecol. 2005, 20, 63–72. [Google Scholar] [CrossRef]
- Chen, R.; Twilley, R.R. A gap dynamic model of mangrove forest development along gradients of soil salinity and nutrient resources. J. Ecol. 1998, 86, 37–51. [Google Scholar] [CrossRef]
- Levin, S.A.; Muller-Landau, H.C.; Nathan, R.; Chave, J. The ecology and the evolution of seed dispersal: A theoretical perspective. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 575–604. [Google Scholar] [CrossRef]
- Van der Stocken, T.; de Ryck, D.J.R.; Vanschoenwinkel, B.; Deboelpaep, E.; Bouma, T.J.; Dahdouh-Guebas, F.; Koedam, N. Impact of landscape structure on propagule dispersal in mangrove forests. Mar. Ecol. Prog. Ser. 2015, 524, 95–106. [Google Scholar] [CrossRef]
- Sousa, W.P.; Kennedy, P.G.; Mitchell, B.J.; Ordóñez, B.M.L. Supply-side ecology in mangroves: Do propagule dispersal and seedling establishment explain forest structure? Ecol. Monogr. 2007, 77, 53–76. [Google Scholar] [CrossRef]
- Van Speybroeck, D. Regeneration strategy of mangroves along the Kenya coast: A first approach. Hydrobiologia 1992, 247, 243–251. [Google Scholar] [CrossRef]
- Cannicci, S.; Burrows, D.; Fratini, S.; Smith, T.J.; Offenberg, J.; Dahdouh-Guebas, F. Faunal impact on vegetation structure and ecosystem function in mangrove forests: A review. Aquat. Bot. 2008, 89, 186–200. [Google Scholar] [CrossRef]
- Dahdouh-Guebas, F.; Koedam, N.; Satyanarayana, B.; Cannicci, S. Human hydrographical changes interact with propagule predation behaviour in Sri Lankan mangrove forests. J. Exp. Mar. Biol. Ecol. 2011, 399, 188–200. [Google Scholar] [CrossRef]
- Tomlinson, P.B. The Botany of Mangroves, 1st ed.; Cambridge University Press: Cambridge, UK, 1994; ISBN 9780521466752. [Google Scholar]
- De Ryck, D.J.R.; Robert, E.M.R.; Schmitz, N.; Van der Stocken, T.; Di Nitto, D.; Dahdouh-Guebas, F.; Koedam, N. Size does matter, but not only size: Two alternative dispersal strategies for viviparous mangrove propagules. Aquat. Bot. 2012, 103, 66–73. [Google Scholar] [CrossRef] [Green Version]
- Van der Stocken, T.; De Ryck, D.J.R.; Balke, T.; Bouma, T.J.; Dahdouh-Guebas, F.; Koedam, N. The role of wind in hydrochorous mangrove propagule dispersal. Biogeosciences 2013, 10, 3635–3647. [Google Scholar] [CrossRef] [Green Version]
- Van der Stocken, T.; Vanschoenwinkel, B.; De Ryck, D.J.; Bouma, T.J.; Dahdouh-Guebas, F.; Koedam, N.; Álvarez, I. Interaction between Water and Wind as a Driver of Passive Dispersal in Mangroves. PLoS ONE 2015, 10, e0121593. [Google Scholar] [CrossRef] [Green Version]
- Van der Stocken, T.; Wee, A.K.S.; de Ryck, D.J.R.; Vanschoenwinkel, B.; Friess, D.A.; Dahdouh-Guebas, F.; Simard, M.; Koedam, N.; Webb, E.L. A general framework for propagule dispersal in mangroves. Biol. Rev. Camb. Philos. Soc. 2019, 94, 1547–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hintze, C.; Heydel, F.; Hoppe, C.; Cunze, S.; König, A.; Tackenberg, O. D3: The Dispersal and Diaspore Database—Baseline data and statistics on seed dispersal. Perspect. Plant Ecol. Evol. Syst. 2013, 15, 180–192. [Google Scholar] [CrossRef] [Green Version]
- Tamme, R.; Götzenberger, L.; Zobel, M.; Bullock, J.M.; Hooftman, D.A.; Kaasik, A.; Pärtel, M. Predicting species’ maximum dispersal distances from simple plant traits. Ecology 2014, 95, 505–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vittoz, P.; Engler, R. Seed dispersal distances: A typology based on dispersal modes and plant traits. Bot. Helv. 2007, 117, 109–124. [Google Scholar] [CrossRef] [Green Version]
- Botkin, D.B.; Janak, J.F.; Wallis, J.R. Rationale, limitations, and assumptions of a northeastern forest growth simulator. IBM J. Res. Dev. 1972, 16, 101–116. [Google Scholar] [CrossRef]
- Shugart, H.H. A Theory of Forest Dynamics: The Ecological Implications of Forest Succession Models; Springer: New York, NY, USA, 1984; ISBN 9783540960003. [Google Scholar]
- Craine, J.M. Resource Strategies of Wild Plants; Princeton University Press: Princeton, NJ, USA, 2009; ISBN 978-0-691-13911-1. [Google Scholar]
- Grams, T.E.E.; Andersen, C.P. Competition for resources in trees: Physiological versus morphological plasticity. In Progress in Botany; Springer: Berlin, Germnay, 2007; pp. 356–381. ISBN 3540368329. [Google Scholar]
- Casper, B.B.; Jackson, R.B. Plant competition underground. Annu. Rev. Ecol. Evol. Syst. 1997, 28, 545–570. [Google Scholar] [CrossRef] [Green Version]
- Huisman, J.; Weissing, F.J. Light-Limited Growth and Competition for Light in Well-Mixed Aquatic Environments: An Elementary Model. Ecology 1994, 75, 507–520. [Google Scholar] [CrossRef] [Green Version]
- Borer, E.T.; Seabloom, E.W.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Lind, E.M.; Adler, P.B.; Alberti, J.; Anderson, T.M.; Bakker, J.D.; et al. Herbivores and nutrients control grassland plant diversity via light limitation. Nature 2014, 508, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Grimm, V.; Berger, U.; Bastiansen, F.; Eliassen, S.; Ginot, V.; Giske, J.; Goss-Custard, J.; Grand, T.; Heinz, S.K.; Huse, G.; et al. A standard protocol for describing individual-based and agent-based models. Ecol. Model. 2006, 198, 115–126. [Google Scholar] [CrossRef]
- Grimm, V.; Berger, U.; DeAngelis, D.L.; Polhill, J.G.; Giske, J.; Railsback, S.F. The ODD protocol: A review and first update. Ecol. Model. 2010, 221, 2760–2768. [Google Scholar] [CrossRef] [Green Version]
- Grimm, V.; Wissel, C. Babel, or the ecological stability discussions: An inventory and analysis of terminology and a guide for avoiding confusion. Oecologia 1997, 109, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.B.; Agnew, A.D.Q.; Roxburgh, S.H. The Nature of Plant Communities; Cambridge University Press: New York, NY, USA, 2019; ISBN 9781108482219. [Google Scholar]
- Lehman, C.L.; Tilman, D. Biodiversity, Stability, and Productivity in Competitive Communities. Am. Nat. 2000, 156, 534–552. [Google Scholar] [CrossRef]
- Gunderson, L.H. Ecological Resilience—In Theory and Application. Annu. Rev. Ecol. Syst. 2000, 31, 425–439. [Google Scholar] [CrossRef] [Green Version]
- Shade, A.; Peter, H.; Allison, S.D.; Baho, D.L.; Berga, M.; Bürgmann, H.; Huber, D.H.; Langenheder, S.; Lennon, J.T.; Martiny, J.B.H.; et al. Fundamentals of microbial community resistance and resilience. Front. Microbiol. 2012, 3, 417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolker, B.M.; Pacala, S.W.; Neuhauser, C. Spatial dynamics in model plant communities: What do we really know? Am. Nat. 2003, 162, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Pacala, S.W.; Silander, J.A., Jr. Field tests of neighborhood population dynamic models of two annual weed species. Ecol. Monogr. 1990, 60, 113–134. [Google Scholar] [CrossRef]
- Dormann, C.F.; Roxburgh, S.H. Experimental evidence rejects pairwise modelling approach to coexistence in plant communities. Proc. R. Soc. B Biol. Sci. 2005, 272, 1279–1285. [Google Scholar] [CrossRef] [Green Version]
- Silvertown, J.; Wilson, J.B. Spatial interactions among grassland plant populations. In The Geometry of Ecological Interactions: Simplifying Spatial Complexity; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2000; pp. 28–47. ISBN 9780521642941. [Google Scholar]
- Loreau, M. From Populations to Ecosystems: Theoretical Foundations for a New Ecological Synthesis; Princeton University Press: Princeton, NJ, USA, 2010; ISBN 1400834163. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Available online: http://www.R-project.org (accessed on 2 June 2013).
- Cortez, P. Modern Optimization with R; Springer: Cham, Switzerland, 2014; ISBN 9783319082622. [Google Scholar]
- Roughgarden, J. Primer of Ecological Theory; Prentice Hall: Upper Saddle River, NJ, USA, 1998; ISBN 9780134420622. [Google Scholar]
- Townsend, C.R.; Begon, M.; Harper, J.L. Essentials of Ecology; Wiley: Hoboken, NJ, USA, 2009; ISBN 9781444305340. [Google Scholar]
- Morin, P.J. Community Ecology, 2nd ed.; Wiley-Blackwell A John Wiley & Sons: Chichester, UK, 2012; ISBN 9781444338218. [Google Scholar]
- Lin, M.; Lucas, H.C.; Shmueli, G. Research Commentary—Too Big to Fail: Large Samples and the p-Value Problem. Inf. Syst. Res. 2013, 24, 906–917. [Google Scholar] [CrossRef] [Green Version]
- Poisot, T. Teaching Isoclines in the Two-Species Competitive Logistic Model. Available online: http://timotheepoisot.fr/2014/03/18/teaching-isoclines/ (accessed on 15 March 2015).
- Milborrow, S. Earth: Multivariate Adaptive Regression Spline Models. R Package Version 3.2-3. Available online: https://CRAN.R-project.org/package=earth12 (accessed on 2 June 2013).
- Warton, D.I.; Duursma, R.A.; Falster, D.S.; Taskinen, S. Smatr 3—An R package for estimation and inference about allometric lines. Methods Ecol. Evol. 2012, 13, 257–259. [Google Scholar] [CrossRef]
- Reineke, L.H. Perfecting a Stand-Density Index for Even-Aged Forests; U.S. G.P.O.: Washington, DC, USA, 1933. [Google Scholar]
- Imbert, D. Hurricane disturbance and forest dynamics in east Caribbean mangroves. Ecosphere 2018, 9, e02231. [Google Scholar] [CrossRef] [Green Version]
- Costanza, R.; Pérez-Maqueo, O.; Martinez, M.L.; Sutton, P.; Anderson, S.J.; Mulder, K. The Value of Coastal Wetlands for Hurricane Protection. AMBI 2008, 37, 241–248. [Google Scholar] [CrossRef]
- Doyle, T.W.; Krauss, K.W.; Wells, C.J. Landscape analysis and pattern of hurricane impact and circulation on mangrove forests of the Everglades. Wetlands 2009, 29, 44–53. [Google Scholar] [CrossRef]
- Frolking, S.; Palace, M.W.; Clark, D.B.; Chambers, J.Q.; Shugart, H.H.; Hurtt, G.C. Forest disturbance and recovery: A general review in the context of spaceborne remote sensing of impacts on aboveground biomass and canopy structure. J. Geophys. Res. 2009, 114. [Google Scholar] [CrossRef]
- Law, R.; Purves, D.W.; Murrell, D.J.; Dieckmann, U. Causes and effects of smal-scale spatial structure in plant populations. In Integrating Ecology and Evolution in a Spatial Context: The 14th Special Symposium of the British Ecological Society Held at Royal Holloway College, University of London, 29-31 August, 2000; Blackwell Scientific: Oxford, UK, 2001; pp. 21–44. ISBN 9780632058242. [Google Scholar]
- Czárán, T. Spatiotemporal Models of Population and Community Dynamics, 1st ed.; Chapman & Hall: London, UK; New York, NY, USA, 1998; ISBN 9780412575501. [Google Scholar]
- Whelan, K.R.T. The Successional Dynamics of Lightning-Initiated Canopy Gaps in the Mangrove Forests of Shark River, Everglades National Park, USA. Ph. D. Thesis, Florida International University, Miami, FL, USA, 2005. [Google Scholar]
- López-Hoffmann, L.; Ackerly, D.D.; Anten, N.P.R.; Denoyer, J.L.; Martinez-Ramos, M. Gap-dependence in mangrove life-history strategies: A consideration of the entire life cycle and patch dynamics. J. Ecol. 2007, 95, 1222–1233. [Google Scholar] [CrossRef]
- Novoplansky, A. Picking battles wisely: Plant behaviour under competition. Plantcell Environ. 2009, 32, 726–741. [Google Scholar] [CrossRef]
- Seidel, D.; Leuschner, C.; Müller, A.; Krause, B. Crown plasticity in mixed forests—Quantifying asymmetry as a measure of competition using terrestrial laser scanning. For. Ecol. Manag. 2011, 261, 2123–2132. [Google Scholar] [CrossRef]
- Barbeito, I.; Collet, C.; Ningre, F. Crown responses to neighbor density and species identity in a young mixed deciduous stand. Trees 2014, 28, 1751–1765. [Google Scholar] [CrossRef]
- Barbeito, I.; Dassot, M.; Bayer, D.; Collet, C.; Drössler, L.; Löf, M.; del Rio, M.; Ruiz-Peinado, R.; Forrester, D.I.; Bravo-Oviedo, A.; et al. Terrestrial laser scanning reveals differences in crown structure of Fagus sylvatica in mixed vs. pure European forests. For. Ecol. Manag. 2017, 405, 381–390. [Google Scholar] [CrossRef]
- Stoll, P.; Newbery, D.M. Evidence of species-specific neighborhood effects in the Dipterocarpaceae of a Bornean rain forest. Ecology 2005, 86, 3048–3062. [Google Scholar] [CrossRef] [Green Version]
- Richards, M.; McDonald, A.J.S.; Aitkenhead, M.J. Optimisation of competition indices using simulated annealing and artificial neural networks. Ecol. Model. 2008, 214, 375–384. [Google Scholar] [CrossRef]
- Pretzsch, H.; Schütze, G. Transgressive overyielding in mixed compared with pure stands of Norway spruce and European beech in Central Europe: Evidence on stand level and explanation on individual tree level. Eur. J. For. Res 2009, 128, 183–204. [Google Scholar] [CrossRef]
- Gilbert, I.R.; Jarvis, P.G.; Smith, H. Proximity signal and shade avoidance differences between early and late successional trees. Nature 2001, 411, 792–795. [Google Scholar] [CrossRef]
- Vincent, G.; Harja, D. Exploring ecological significance of tree crown plasticity through three-dimensional modelling. Ann. Bot. 2007, 101, 1221–1231. [Google Scholar] [CrossRef] [Green Version]
- Longuetaud, F.; Piboule, A.; Wernsdörfer, H.; Collet, C. Crown plasticity reduces inter-tree competition in a mixed broadleaved forest. Eur. J. For. Res. 2013, 132, 621–634. [Google Scholar] [CrossRef]
- Ligot, G.; Balandier, P.; Fayolle, A.; Lejeune, P.; Claessens, H. Height competition between Quercus petraea and Fagus sylvatica natural regeneration in mixed and uneven-aged stands. For. Ecol. Manag. 2013, 304, 391–398. [Google Scholar] [CrossRef]
- Remmert, H. The Mosaic-Cycle Concept of Ecosystems; Springer: Berlin/Heidelberg, Germany, 1991; ISBN 9783642756528. [Google Scholar]
- Vovides, A.G.; Berger, U.; Grueters, U.; Guevara, R.; Pommerening, A.; Lara-Domínguez, A.L.; López-Portillo, J. Change in drivers of mangrove crown displacement along a salinity stress gradient. Funct. Ecol. 2018, 32, 2753–2765. [Google Scholar] [CrossRef] [Green Version]
- Silvertown, J. Plant coexistence and the niche. Trends Ecol. Evol. 2004, 19, 605–611. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Hillebrand, H.; Harpole, W.S.; Gross, K.; Ptacnik, R. Separating the influence of resource ‘availability’ from resource ‘imbalance’ on productivity-diversity relationships. Ecol. Lett. 2009, 12, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.B. The twelve theories of co-existence in plant communities: The doubtful, the important and the unexplored. J. Veg. Sci. 2011, 22, 184–195. [Google Scholar] [CrossRef]
- Tilman, D. The resource-ratio hypothesis of plant succession. Am. Nat. 1985, 125, 827–852. [Google Scholar] [CrossRef]
- Wilson, J.B. Plant species richness: The world records. J. Veg. Sci. 2012, 23, 796–802. [Google Scholar] [CrossRef]
- Peñuelas, J.; Terradas, J.; Lloret, F. Solving the conundrum of plant species coexistence: Water in space and time matters most. New Phytol. 2011, 189, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D. Competition and biodiversity in spatially structured habitats. Ecol. Brooklyn 1994, 75, 2–16. [Google Scholar] [CrossRef]
- Clark, J.S. Fecundity of trees and the colonization-competition hypothesis. Ecol. Monogr. 2004, 74, 415–442. [Google Scholar] [CrossRef]
- Chesson, P. Multispecies competition in variable environments. Theor. Popul. Biol. 1994, 45, 227–276. [Google Scholar] [CrossRef]
- Gleeson, S.K.; Tilman, D. Plant Allocation and the Multiple Limitation Hypothesis. Am. Nat. 1992, 139, 1322–1343. [Google Scholar] [CrossRef]
- Dutta, P.S.; Kooi, B.W.; Feudel, U. Multiple resource limitation: Nonequilibrium coexistence of species in a competition model using a synthesizing unit. Theor. Ecol. 2014, 7, 407–421. [Google Scholar] [CrossRef]
- Harpole, W.S.; Tilman, D. Grassland species loss resulting from reduced niche dimension. Nature 2007, 446, 791–793. [Google Scholar] [CrossRef] [PubMed]








| a | Treatment | r1 (×10−2) [m3 ha−1 yr−1] | K1 [m3 ha−1] | V0 [m3 ha−1] | RSS (×10−3) | ||
| Random Dispersal | 5.119 | 752.42 | 0.66 | 6.76 | |||
| Natural Dispersal | 5.201 | 878.35 | 31.93 | 46.76 | |||
| b | Treatment | K1 [indiv. ha−1] | K1 [m3 ha−1] | α11,indiv (×10−4) | α11 (×10−4) | ||
| Random Dispersal | 822.02 | 621.72 | 1.98 | 2.62 | |||
| Natural Dispersal | 831.54 | 617.42 | 1.93 | 2.60 | |||
| c | Treatment | α11 (slope) (×10−4) | α11 (intercept) | R2 | α1<-2 (slope) (×10−4) | α1<-2 (intercept) | R2 |
| Plasticity | Random Dispersal | 2.92 | 0.002 | 0.922 | 1.93 | 0.007 | 0.932 |
| Natural Dispersal | 1.75 | 0.066 | 0.874 | 1.41 | 0.001 | 0.918 | |
| Treatment | α22 (slope) (×10−4) | α22 (intercept) | R2 | α2<-1 (slope) (×10−4) | α2<-1 (intercept) | R2 | |
| Random Dispersal | 2.92 | 0.003 | 0.916 | 2.08 | 0.002 | 0.937 | |
| Natural Dispersal | 1.66 | 0.069 | 0.869 | 1.36 | 0.002 | 0.938 | |
| d | Treatment | α11 (slope) (×10−4) | α11 (intercept) | R2 | α1<-2 (slope) (×10−4) | α1<-2 (intercept) | R2 |
| Relaxation | Random Dispersal | 2.67 | −0.005 | 0.980 | 2.60 | 0.003 | 0.968 |
| Natural Dispersal | 1.77 | 0.054 | 0.929 | 1.84 | 0.001 | 0.944 | |
| Treatment | α22 (slope) (×10−4) | α22 (intercept) | R2 | α2<-1 (slope) (×10−4) | α2<-1 (intercept) | R2 | |
| Random Dispersal | 2.66 | −0.004 | 0.977 | 2.60 | 0.003 | 0.977 | |
| Natural Dispersal | 1.77 | 0.053 | 0.933 | 1.81 | 0.001 | 0.938 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grueters, U.; Ibrahim, M.R.; Schmidt, H.; Tiebel, K.; Horn, H.; Pranchai, A.; Vovides, A.G.; Vogt, J.; Otero, V.; Satyanarayana, B.; et al. Stable Coexistence in a Field-Calibrated Individual-Based Model of Mangrove Forest Dynamics Caused by Inter-Specific Crown Plasticity. Forests 2021, 12, 955. https://doi.org/10.3390/f12070955
Grueters U, Ibrahim MR, Schmidt H, Tiebel K, Horn H, Pranchai A, Vovides AG, Vogt J, Otero V, Satyanarayana B, et al. Stable Coexistence in a Field-Calibrated Individual-Based Model of Mangrove Forest Dynamics Caused by Inter-Specific Crown Plasticity. Forests. 2021; 12(7):955. https://doi.org/10.3390/f12070955
Chicago/Turabian StyleGrueters, Uwe, Mohd Rodila Ibrahim, Hartmut Schmidt, Katharina Tiebel, Hendrik Horn, Aor Pranchai, Alejandra G. Vovides, Juliane Vogt, Viviana Otero, Behara Satyanarayana, and et al. 2021. "Stable Coexistence in a Field-Calibrated Individual-Based Model of Mangrove Forest Dynamics Caused by Inter-Specific Crown Plasticity" Forests 12, no. 7: 955. https://doi.org/10.3390/f12070955