The Effect of Sucrose Supplementation on the Micropropagation of Salix viminalis L. Shoots in Semisolid Medium and Temporary Immersion Bioreactors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Micropropagation in Semisolid and Liquid Medium
2.2. Growth Conditions and Frequency of Immersion
2.3. Culture System and Sucrose Supplementation
2.4. Explant Type
2.5. Acclimatization
2.6. Data Recording and Statistical Analysis
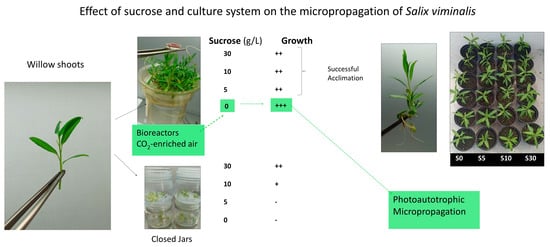
3. Results
3.1. Growth Conditions and Frequency of Immersion
3.2. Culture System and Sucrose Supplementation
3.3. Explant Type
3.4. Acclimatization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Etienne, H.; Berthouly, M. Temporary immersion systems in plant micropropagation. Plant Cell Tissue Organ Cult. 2002, 69, 215–231. [Google Scholar] [CrossRef]
- Watt, M.P. The status of temporary immersion system (TIS) technology for plant micropropagation. Afr. J. Biotechn. 2012, 11, 14025–14035. [Google Scholar] [CrossRef]
- Georgiev, V.; Schumann, A.; Pavlov, A.; Bley, T. Temporary immersion systems in plant biotechnology. Eng. Life Sci. 2014, 14, 607–621. [Google Scholar] [CrossRef]
- Valdiani, A.; Hansen, O.K.; Nielsen, U.B.; Johannsen, V.K.; Shariat, M.; Georgiev, M.I.; Omidvar, V.; Ebrahimi, M.; Dinanai, E.T.; Abiri, R. Bioreactor-based advances in plant tissue and cell culture: Challenges and prospects. Crit. Rev. Biotechnol. 2019, 39, 20–34. [Google Scholar] [CrossRef]
- Vidal, N.; Sánchez, C. Use of bioreactor systems in the propagation of forest trees. Eng. Life Sci. 2019, 19, 896–915. [Google Scholar] [CrossRef] [Green Version]
- Regueira, M.; Rial, E.; Blanco, B.; Bogo, B.; Aldrey, A.; Correa, B.; Varas, E.; Sánchez, C.; Vidal, N. Micropropagation of axillary shoots of Salix viminalis using a temporary immersion system. Trees 2018, 32, 61–71. [Google Scholar] [CrossRef]
- Xiao, Y.; Niu, G.; Kozai, T. Development and application of photoautotrophic micropropagation plant system. Plant Cell Tissue Organ Cult. 2011, 105, 149–158. [Google Scholar] [CrossRef]
- Mleczek, M.; Rutkowski, P.; Rissmann, I.; Kaczmarek, Z.; Golinski, P.; Szentner, K.; Strażyńska, K.; Stachowiak, A. Biomass productivity and phytoremediation potential of Salix alba and Salix viminalis. Biomass Bioenerg. 2010, 34, 1410–1418. [Google Scholar] [CrossRef]
- Berlin, S.; Trybush, S.O.; Fogelqvist, J.; Gyllenstrand, N.; Hallingbaeck, H.R.; Åhman, I.; Nordh, N.-E.; Shield, I.; Powers, S.J.; Weih, M.; et al. Genetic diversity, population structure and phenotypic variation in European Salix viminalis L. (Salicaceae). Tree Genet. Genom. 2014, 10, 1595–1610. [Google Scholar] [CrossRef] [Green Version]
- Scriba, C.; Lunguleasa, A.; Spirchez, C.; Ciobanu, V. Influence of INGER and TORDIS energetic willow clones planted on contaminated soil on the survival rates, yields and calorific value. Forests 2021, 12, 826. [Google Scholar] [CrossRef]
- Touceda-González, M.; Álvarez-López, V.; Prieto-Fernández, A.; Rodríguez-Garrido, B.; Trasar-Cepeda, C.; Mench, M.; Puschenreiter, M.; Quintela-Sabarís, C.; Macías-García, F.; Kidd, P.S. Aided phytostabilisation reduces metal toxicity, improves soil fertility and enhances microbial activity in Cu-rich mine tailings. J. Environ. Manag. 2017, 186, 301–313. [Google Scholar] [CrossRef]
- Palomo-Ríos, E.; Macalpine, E.; Shield, I.; Amey, J.; Karaoğlu, C.; West, J.; Hanley, S.; Krygier, R.; Karp, A.; Jones, H.D. Efficient method for rapid multiplication of clean and healthy willow clones via in vitro propagation with broad genotype applicability. Can. J. For. Res. 2015, 45, 1662–1667. [Google Scholar] [CrossRef] [Green Version]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Miyashita, Y.; Kitaya, Y.; Kubota, C.; Kozai, T. Photoautotrophic growth of potato plantlets as affected by explant leaf area, fresh weight and stem length. Sci. Hortic. 1996, 65, 199–202. [Google Scholar] [CrossRef]
- Cuenca Valera, B.; Aldrey Villar, A.; Blanco Beiro, B.; Vidal González, N. Use of a Continuous Immersion System (CIS) for micropropagation of chestnut in photoautotrophic and photomixotrophic conditions. In Woody Plant Production Integrating Genetic and Vegetative Propagation Technologies, Proceedings of the 3rd International Conference of the IUFRO Unit 2.09.02, Vitoria-Gasteiz, Spain, 8–12 September 2014; Park, Y.S., Bonga, J.M., Eds.; IUFRO: Stockholm, Sweden, 2015; pp. 112–116. [Google Scholar]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- IBM SPSS Statistics. Available online: https://www.ibm.com/products/spss-statistics (accessed on 30 September 2021).
- McAlister, B.; Finnie, J.; Watt, M.P.; Blakeway, F. Use of temporary immersion system (RITA®) for production of commercial Eucalyptus clones in Mondi Forests (SA). Plant Cell Tissue Organ Cult. 2005, 81, 347–358. [Google Scholar] [CrossRef]
- García-Ramírez, Y.; Barrera, G.P.; Seijo, M.F.; Barbón, R.; Concepción-Hernández, M.; Mendoza-Rodríguez, M.F.; Torres-García, S. Effect of sucrose on physiological and biochemical changes of proliferated shoots of Bambusa vulgaris Schrad. Ex Wendl in temporary immersion. Plant Cell Tissue Organ Cult. 2019, 137, 239–247. [Google Scholar] [CrossRef]
- Zhu, L.H.; Li, X.Y.; Welander, M. Optimisation of growing conditions for the apple rootstock M26 grown in RITA® containers using temporary immersion principle. Plant Cell Tissue Organ Cult. 2005, 81, 313–318. [Google Scholar] [CrossRef]
- Latawa, Y.; Shukla, M.R.; Saxena, P.K. An efficient temporary immersion system for micropropagation of hybrid hazelnut. Botany 2016, 94, 1–8. [Google Scholar] [CrossRef]
- Thi, L.T.; Park, Y.G.; Jeong, B.R. Growth and development of carnation ‘Dreambyul’ plantlets in a temporary immersion system and comparisons with conventional solid culture methods. In Vitro Cell. Dev. Biol. Plant 2019, 55, 539–548. [Google Scholar] [CrossRef]
- Arano-Avalos, S.; Gómez-Merino, F.C.; Mancilla-Álvarez, E.; Sánchez-Páez, R.; Bello-Bello, J.J. An efficient protocol for comercial micropropagation of malanga (Colocasia esculenta L. Schott) using temporary immersion. Sci. Hortic. 2020, 261, 108998:1–108998:6. [Google Scholar] [CrossRef]
- Murch, S.J.; Chunzhao, L.; Romero, R.M.; Saxena, P.K. In vitro culture and temporary immersion bioreactor production of Crescentia cujete. Plant Cell Tissue Organ Cult. 2004, 78, 36–68. [Google Scholar] [CrossRef]
- Deng, Z.; Chu, J.; Wang, Q.; Wang, L. Effect of different carbon sources on the accumulation of carbohydrate, nutrient absorption and the survival rate of Chinese Ash (Fraxinus mandshurica) explants in vitro. Afr. J. Agr. Res. 2012, 7, 3111–3119. [Google Scholar] [CrossRef]
- Quiala, E.; Cañal, M.J.; Meijón, M.; Rodriguez, R.; Chávez, M.; Valledor, L.; de Feria, M.; Barbón, R. Morphological and physiological responses of proliferating shoots of teak to temporary immersion and BA treatments. Plant Cell Tissue Organ Cult. 2012, 109, 223–234. [Google Scholar] [CrossRef] [Green Version]
- Luna, C.V.; Gonzalez, A.M.; Mroginski, L.A.; Sansberro, P.A. Anatomical and histological features of Ilex paraguariensis leaves under different in vitro shoot culture systems. Plant Cell Tissue Organ Cult. 2017, 129, 457–467. [Google Scholar] [CrossRef]
- Kim, N.-Y.; Hwang, H.-D.; Kim, J.-H.; Kwon, B.-M.; Kim, D.; Park, S.-Y. Efficient production of virus-free apple plantlets using the temporary immersion bioreactor system. Hortic. Environ. Biotechnol. 2020, 61, 779–785. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Xiao, Y.; Kozai, T. Photoautotrophic Micropropagation. In Plant Factory–An Indoor Vertical Farming System for Efficient Quality Food Production, 2nd ed.; Kozai, T., Niu, G., Takagaki, M., Eds.; Elsevier, Academic Press: New York, NY, USA, 2020; Chapter 23; pp. 333–346. [Google Scholar] [CrossRef]
- Sha Valli Khan, P.S.; Kozai, T.; Nguyen, Q.T.; Kubota, C.; Dhawan, V. Growth and net photosynthetic rates of Eucalyptus tereticornis Smith under photomixotrophic and various photoautotrophic micropropagation conditions. Plant Cell Tissue Organ Cult. 2002, 71, 141–146. [Google Scholar] [CrossRef]
- Habibi, N.; Purohit, S.D. Photosynthetic efficiency and in vitro growth of Celastrus paniculatus Willd. under varied concentrations of CO2. Int. J. Phytocosmetics Nat. Ingred. 2019, 6, 11:1–11:10. [Google Scholar] [CrossRef]
- Fortini, E.A.; Batista, D.S.; Memedes-Rodrigues, T.C.; Felipe, S.H.S.; Corriera, L.N.F.; Chagas, K.; Silva, P.O.; Rocha, D.I.; Otoni, W.C. Gas exchange rates and sucrose concentrations affect plant growth and production of flavonoids in Vernonia condensate grown in vitro. Plant Cell Tissue Organ Cult. 2021, 144, 593–605. [Google Scholar] [CrossRef]
- Sha Valli Khan, P.S.; Kozai, T.; Nguyen, Q.T.; Kubota, C.; Dhawan, V. Growth and water relations of Paulownia fortunei under photomixotrophic and photoautotrophic conditions. Biol. Plant. 2003, 46, 161–166. [Google Scholar] [CrossRef]
- Mosaleeyanon, K.; Cha-um, S.; Kirdmanee, C. Enhanced growth and photosynthesis of rain tree (Samanea saman Merr.) plantlets in vitro under a CO2-enriched condition with decreased sucrose concentrations in the medium. Sci. Hortic. 2004, 103, 51–63. [Google Scholar] [CrossRef]
- Kirdmanee, C.; Kitaya, Y.; Kozai, T. Effects of CO2 enrichment and supporting material in vitro on photoautotrophic growth of Eucalyptus plantlets in vitro and ex vitro. In Vitro Cell. Dev. Biol. Plant 1995, 31, 144–149. [Google Scholar] [CrossRef]
- Zobayed, S.M.A.; Afreen-Zobayed, F.; Kubota, C.; Kozai, T. Mass propagation of Eucalyptus camaldulensis in a scaled-up vessel under in vitro photoautotrophic condition. Ann. Bot. 2000, 85, 587–592. [Google Scholar] [CrossRef] [Green Version]
- Zobayed, S.M.A.; Afreen, F.; Kozai, T. Physiology of eucalyptus plantlets grown photoautotrophically in a scaled-up vessel. In Vitro Cell. Dev. Biol. Plant 2001, 37, 807–813. [Google Scholar] [CrossRef]
- Tanaka, M.; Giang, D.T.T.; Murakami, A. Application of a novel disposable film culture system to photoautotrophic micropropagation of Eucalyptus uro-grandis (Urophylia x grandis). In Vitro Cell. Dev. Biol. Plant 2005, 41, 173–180. [Google Scholar] [CrossRef]
- Cha-Um, S.; Chanseetis, C.; Chintakovid, W.; Pichakum, A.; Supaibulwatana, K. Promoting root induction and growth of in vitro macadamia (Macadamia tetraphylla L. ‘Keaau’) plantlets using CO2-enriched photoautotrophic conditions. Plant Cell Tissue Organ Cult. 2011, 106, 435–444. [Google Scholar] [CrossRef]
- Vidal, N.; Blanco, B.; Cuenca, B. A temporary immersion system for micropropagation of axillary shoots of hybrid chestnut. Plant Cell Tissue Organ Cult. 2015, 123, 229–243. [Google Scholar] [CrossRef]
- Vidal, N.; Aldrey, A.; Blanco, B.; Correa, B.; Sánchez, C.; Cuenca, B. Proliferation and rooting of chestnut under photoautotrophic conditions. In Development and Application of Vegetative Propagation Technologies in Plantation Forestry to Cope with A Changing Climate and Environment, Proceedings of the 4th International Conference of the IUFRO Unit 2.09.02, La Plata, Argentina, 19–23 September 2016; Bonga, J.M., Park, Y.-S., Trontin, J.-F., Eds.; IUFRO: Stockholm, Sweden, 2017; pp. 119–127. [Google Scholar]
- Zobayed, S.M.A. Ventilation in micropropagation. In Photoautotrophic (Sugar-Free Medium) Micropropagation as a New Micropropagation and Transplant Production System; Kozai, T., Afreen, F., Zobayed, S.M.A., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 147–186. [Google Scholar] [CrossRef]
- Lucchesini, M.; Monteforti, G.; Mensuali-Sodi, A.; Serra, G. Leaf ultrastructure, photosynthetic rate and growth of myrtle plantlets under different in vitro culture conditions. Biol. Plant 2006, 50, 161–168. [Google Scholar] [CrossRef]
- Sáez, P.; Bravo, L.; Latsague, M.; Sanchez-Olate, M.; Ríos, D. Increased light intensity during in vitro culture improves water loss control and photosynthetic performance of Castanea sativa grown in ventilated vessels. Sci. Hortic. 2012, 130, 7–16. [Google Scholar] [CrossRef]
- Ševčíková, H.; Lhotáková, Z.; Hamet, J.; Lipavská, H. Mixotrophic in vitro cultivations: The way to go astray in plant physiology. Physiol. Plant. 2019, 167, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Gago, J.; Daloso, D.M.; Carriquí, M.; Nadal, M.; Morales, M.; Araújo, W.L.; Nunes-Nesi, A.; Flexas, J. Mesophyll conductance: The leaf corridors for photosynthesis. Biochem. Soc. Trans. 2020, 48, 429–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gago, J.; Daloso, D.M.; Carriquí, M.; Nadal, M.; Morales, M.; Araújo, W.L.; Nunes-Nesi, A.; Perera-Castro, A.V.; Clemente-Moreno, M.J.; Flexas, J. The photosynthesis game is in the ‘inter-play’: Mechanisms underlying CO2 diffusion in leaves. Environ. Exp. Bot. 2020, 178, 104174:1–104174:15. [Google Scholar] [CrossRef]
- Douthe, C.; Gago, J.; Ribas-Carbó, M.; Núñez, R.; Pedrol, N.; Flexas, J. Measuring photosynthesis and respiration with infrared gas analysers. In Advances in Plant Ecophysiology Techniques; Sánchez-Moreiras, A.M., Reigosa, M.J., Eds.; Springer: Berlin, Germany, 2018; pp. 51–75. [Google Scholar] [CrossRef]
- Capellades, M.; Vanderschaeghe, A.; Lemeur, R.; Debergh, P. How important is photosynthesis in micropropagation? In The Impact of Biotechnology in Agriculture; Sangwan, R.S., Sangwan-Norreel, B.S., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1990; pp. 29–38. [Google Scholar] [CrossRef]
- Serret, M.D.; Trillas, M.I.; Mates, J.; Araus, J.L. The effect of different closure types, light and sucrose concentrations on carbon isotope composition and growth of Gardenia jasminoides plantlets during micropropagation and subsequent acclimation ex vitro. Plant Cell Tissue Organ Cult. 1997, 47, 217–230. [Google Scholar] [CrossRef]
- Van Huylenbroeck, J.M.; Piqueras, A.; Debergh, P.C. Photosynthesis and carbon metabolism in leaves formed prior and during ex vitro acclimatization of micropropagated plants. Plant Sci. 1998, 134, 21–30. [Google Scholar] [CrossRef]
- De la Viña, G.; Pliego-Alfaro, F.; Driscoll, S.P.; Mitchell, V.J.; Parry, M.A.; Lawlor, D.W. Effects of CO2 and sugars on photosynthesis and composition of avocado leaves grown in vitro. Plant Physiol. Biochem. 1999, 37, 587–595. [Google Scholar] [CrossRef]
- Premkumar, A.; Mercado, J.A.; Quesada, M.A. Effects of in vitro tissue culture conditions and acclimatization on the contents of rubisco, leaf soluble proteins, photosynthetic pigments, and C/N ratio. J. Plant Physiol. 2001, 158, 835–840. [Google Scholar] [CrossRef] [Green Version]
- Arencibia, A.D.; Gómez, A.; Poblete, M.; Vergara, C. High-performance micropropagation of dendroenergetic poplar hybrids in photomixotrophic Temporary Immersion Bioreactors (TIBs). Ind. Crop. Prod. 2017, 96, 102–109. [Google Scholar] [CrossRef]
- Posada-Pérez, L.; Padrón, Y.M.; González, J.O.; Rodríguez, R.S.; Norman, O.M.; Barbón, R.R.; Hurtado, O.R.; Rodríguez, R.C.E.; Daniels, D.; Gómez-Kosky, R. Effects of different culture conditions (photoautotrophic, photomixotrophic) and the auxin indole-butyric acid on the in vitro acclimatization of papaya (Carica papaya L. var. Red Maradol) plants using zeolite as support. Afr. J. Biotechnol. 2015, 14, 2622–2635. [Google Scholar] [CrossRef]
- Gago, J.; Landín, M.; Gallego, P.P. A neurofuzzy logic approach for modeling plant processes: A practical case of in vitro direct rooting and acclimatization of Vitis vinifera L. Plant Sci. 2010, 179, 241–249. [Google Scholar] [CrossRef]
- Gago, J.; Martínez-Núñez, L.; Landín, M.; Flexas, J.; Gallego, P.P. Modeling the effects of light and sucrose on in vitro propagated plants: A multiscale system analysis using artificial intelligence technology. PLoS ONE 2014, 9, e85989:1–e85989:11. [Google Scholar] [CrossRef]
- Hoang, N.N.; Kitaya, Y.; Morishita, T.; Endo, R.; Shibuya, T. A comparative study on growth and morphology of wasabi plantlets under the influence of the micro-environment in shoot and root zones during photoautotrophic and photomixotrophic micropropagation. Plant Cell Tissue Organ Cult. 2017, 130, 255–263. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gago, D.; Vilavert, S.; Bernal, M.Á.; Sánchez, C.; Aldrey, A.; Vidal, N. The Effect of Sucrose Supplementation on the Micropropagation of Salix viminalis L. Shoots in Semisolid Medium and Temporary Immersion Bioreactors. Forests 2021, 12, 1408. https://doi.org/10.3390/f12101408
Gago D, Vilavert S, Bernal MÁ, Sánchez C, Aldrey A, Vidal N. The Effect of Sucrose Supplementation on the Micropropagation of Salix viminalis L. Shoots in Semisolid Medium and Temporary Immersion Bioreactors. Forests. 2021; 12(10):1408. https://doi.org/10.3390/f12101408
Chicago/Turabian StyleGago, Diego, Saladina Vilavert, María Ángeles Bernal, Conchi Sánchez, Anxela Aldrey, and Nieves Vidal. 2021. "The Effect of Sucrose Supplementation on the Micropropagation of Salix viminalis L. Shoots in Semisolid Medium and Temporary Immersion Bioreactors" Forests 12, no. 10: 1408. https://doi.org/10.3390/f12101408