Analytical Pyrolysis of Soluble Bio-Tar from Steam Pretreatment of Bamboo by Using TG–FTIR and Py–GC/MS
Abstract
:1. Introduction
2. Materials and Methods
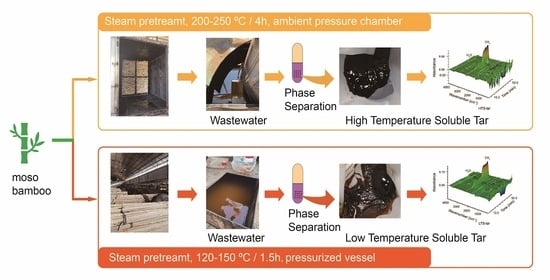
2.1. Materials
2.2. FTIR Analysis of Soluble Bio-Tars in Wastewater
2.3. TG–FTIR Analysis of Soluble Bio-Tars in Wastewater
2.4. Py–GC/MS Analysis of Soluble Bio-Tars in Wastewater
3. Results and Discussion
3.1. FTIR Spectroscopy Analysis of Soluble Bio-Tars from Low and High-Temperature Pretreatment
3.2. TG/DTG Analysis of Soluble Bio-Tars from Low and High-Temperature Pretreatment
3.3. Evolution of Released Gases from Pyrolysis of Soluble Bio-Tars from Low and High-Temperature Pretreatment
3.4. Py–GC/MS Analysis of Soluble Bio-Tars from Low and High-Temperature Pretreatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Octave, S.; Thomas, D. Biorefinery: Toward an Industrial Metabolism. Biochimie 2009, 91, 659–664. [Google Scholar] [CrossRef]
- Xiu, S.; Zhang, B.; Shahbazi, A. Biorefinery Processes for Biomass Conversion to Liquid Fuel. In Biofuel’s Engineering Process Technology; Bernardes, M.A.d.S., Ed.; IntechOpen: Rijeka, Croatia, 2011; Chapter 8. [Google Scholar]
- Liao, Y.; Koelewijn, S.F.; van den Bossche, G.; van Aelst, J.; van den Bosch, S.; Renders, T.; Navare, K.; Nicolaï, T.; van Aelst, K.; Maesen, M.; et al. A Sustainable Wood Biorefinery for Low-Carbon Footprint Chemicals Production. Science 2020, 367, 1385–1390. [Google Scholar] [CrossRef]
- Hu, X.; Gholizadeh, M. Progress of the Applications of Bio-Oil. Renew. Sustain. Energy Rev. 2020, 134, 110124. [Google Scholar] [CrossRef]
- Hu, M.; Zhu, G.; Chen, Y.; Xie, G.; Zhu, M.; Lv, T.; Xu, L. Enhanced Co-Pyrolysis of Corn Stalk and Bio-Tar into Phenolic-Rich Biooil: Kinetic Analysis and Product Distributions. J. Anal. Appl. Pyrolysis 2024, 177, 106358. [Google Scholar] [CrossRef]
- Demirbas, A. Effect of Initial Moisture Content on the Yields of Oily Products from Pyrolysis of Biomass. J. Anal. Appl. Pyrolysis 2004, 71, 803–815. [Google Scholar] [CrossRef]
- Saad, A.; Ratanawilai, S.; Tongurai, C. Catalytic Conversion of Pyrolysis Tar to Produce Green Gasoline-Range Aromatics. Energy Procedia 2015, 79, 471–479. [Google Scholar] [CrossRef]
- Lis, T.; Korzec, N.; Frohs, W.; Tomala, J.; Frączek-Szczypta, A.; Błażewicz, S. Wood-Derived Tar as a Carbon Binder Precursor for Carbon and Graphite Technology. J. Wood Chem. Technol. 2016, 36, 393–400. [Google Scholar] [CrossRef]
- Tu, R.; Sun, Y.; Wu, Y.; Fan, X.; Wang, J.; Cheng, S.; Jia, Z.; Jiang, E.; Xu, X. Bio-Tar-Derived Porous Carbon with High Gas Uptake Capacities. Renew. Energy 2021, 167, 82–90. [Google Scholar] [CrossRef]
- Jia, J.; Sun, Y.; Liu, Z.; Liu, Z.; Huo, L.; Kang, K.; Zhao, Y.; Zhao, L.; Xie, T.; Cao, M.; et al. Waste Bio-Tar Based N-Doped Porous Carbon for Supercapacitors under Dual Activation: Performance, Mechanism, and Assessment. Biochar 2023, 5, 91. [Google Scholar] [CrossRef]
- Yanik, J.; Kornmayer, C.; Saglam, M.; Yüksel, M. Fast Pyrolysis of Agricultural Wastes: Characterization of Pyrolysis Products. Fuel Process. Technol. 2007, 88, 942–947. [Google Scholar] [CrossRef]
- Devi, L.; Ptasinski, K.J.; Janssen, F.J.J.G. A Review of the Primary Measures for Tar Elimination in Biomass Gasiÿcation Processes. Biomass Bioenergy 2003, 24, 125–140. [Google Scholar] [CrossRef]
- Zhang, R.; Brown, R.C.; Suby, A.; Cummer, K. Catalytic Destruction of Tar in Biomass Derived Producer Gas. Energy Convers. Manag. 2004, 45, 995–1014. [Google Scholar] [CrossRef]
- Evans, R.J.; Milne, T.A. Chemistry of Tar Formation and Maturation in the Thermochemical Conversion of Biomass. In Developments in Thermochemical Biomass Conversion: Volume 1/Volume 2; Bridgwater, A.V., Boocock, D.G.B., Eds.; Springer: Dordrecht, The Netherlands, 1997; pp. 803–816. ISBN 978-94-009-1559-6. [Google Scholar]
- Anca-Couce, A.; Brunner, T.; Kanzian, W.; Obernberger, I.; Trattner, K. Characterization and Condensation Behaviour of Gravimetric Bio-tars Produced during Spruce Torrefaction. J. Anal. Appl. Pyrolysis 2016, 119, 173–179. [Google Scholar] [CrossRef]
- Song, K.; Zhang, H.; Wu, Q.; Zhang, Z.; Zhou, C.; Zhang, Q.; Lei, T. Structure and Thermal Properties of Tar from Gasification of Agricultural Crop Residue. J. Therm. Anal. Calorim. 2015, 119, 27–35. [Google Scholar] [CrossRef]
- Ku, C.S.; Mun, S.P. Characterization of Pyrolysis Tar Derived from Lignocellulosic Biomass. J. Ind. Eng. Chem. 2006, 12, 853–861. [Google Scholar]
- Mun, S.P.; Ku, C.S. Pyrolysis GC-MS Analysis of Bio-tars Formed during the Aging of Wood and Bamboo Crude Vinegars. J. Wood Sci. 2010, 56, 47–52. [Google Scholar] [CrossRef]
- Dieguez-Alonso, A.; Anca-Couce, A.; Zobel, N. On-Line Tar Characterization from Pyrolysis of Wood Particles in a Technical-Scale Fixed-Bed Reactor by Applying Laser-Induced Fluorescence (LIF). J. Anal. Appl. Pyrolysis 2013, 102, 33–46. [Google Scholar] [CrossRef]
- Xiong, Z.; Han, H.; Azis, M.M.; Hu, X.; Wang, Y.; Su, S.; Hu, S.; Xiang, J. Formation of the Heavy Tar during Bio-Oil Pyrolysis: A Study Based on Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Fuel 2019, 239, 108–116. [Google Scholar] [CrossRef]
- Li, C.; Suzuki, K. Tar Property, Analysis, Reforming Mechanism and Model for Biomass Gasification—An Overview. Renew. Sustain. Energy Rev. 2009, 13, 594–604. [Google Scholar] [CrossRef]
- Li, C.; Suzuki, K. Resources, Properties and Utilization of Tar. Resour. Conserv. Recycl. 2010, 54, 905–915. [Google Scholar] [CrossRef]
- Valderrama Rios, M.L.; González, A.M.; Lora, E.E.S.; Almazán del Olmo, O.A. Reduction of Tar Generated during Biomass Gasification: A Review. Biomass Bioenergy 2018, 108, 345–370. [Google Scholar] [CrossRef]
- Lee, S.B.; Fasina, O. TG-FTIR Analysis of Switchgrass Pyrolysis. J. Anal. Appl. Pyrolysis 2009, 86, 39–43. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, Z.; Fei, B.; Cai, Z.; Yu, Y.; Liu, X. The Pyrolysis Characteristics of Moso Bamboo. J. Anal. Appl. Pyrolysis 2012, 94, 48–52. [Google Scholar] [CrossRef]
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic Biomass Pyrolysis Mechanism: A State-of-the-Art Review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]






| R.T. (min) | Name of Compound | Formula | Bamboo (Area %) | LTS-Tar * (Area %) | HTS-Tar * (Area %) |
|---|---|---|---|---|---|
| 1.456 | Carbon dioxide | CO2 | 10.09 | 25.60 | 6.56 |
| 1.502 | Acetaldehyde | C2H4O | / | / | 1.57 |
| 1.538 | Trimethyl amine | C3H9N | / | 6.77 | 2.89 |
| 1.594 | Isopropenyl acetate | C5H8O2 | 1.41 | / | / |
| 1.597 | Butanedial | C4H6O2 | 2.41 | 9.03 | 1.59 |
| 1.789 | Acetic acid | C2H4O2 | 11.59 | 11.34 | 2.26 |
| 2.023 | Hydroxyacetone | C3H6O2 | 3.97 | 3.81 | 0.45 |
| 2.234 | 2,5-Dimethylfuran | C6H8O | / | 1.38 | / |
| 2.559 | 1,2-Ethanediol,1-acetate | C4H8O3 | 2.26 | / | / |
| 2.687 | Methyl pyruvate | C4H6O3 | 0.99 | 1.19 | / |
| 3.082 | Furfural | C5H4O2 | 1.57 | 2.17 | / |
| 3.249 | Furfuryl alcohol | C5H6O2 | / | 1.25 | / |
| 3.809 | Dihydropyran | C5H8O | / | 1.05 | / |
| 4.284 | 5-Methyl furfural | C6H6O2 | / | 1.15 | / |
| 4.442 | Phenol | C6H6O | 0.92 | 2.59 | 1.11 |
| 4.638 | Pyridine, 3-methoxy- | C6H7NO | / | / | 1.00 |
| 4.921 | Methyl cyclopentenolone | C6H8O2 | 1.04 | / | / |
| 4.923 | 3-Methyl-1,2-cyclopentanedione | C6H8O2 | / | 1.06 | / |
| 5.196 | N-Carbobenzyloxy-L-glutamine | C13H16N2O5 | / | 1.06 | / |
| 5.387 | Isobutyric anhydride | C8H14O3 | / | / | 7.60 |
| 5.388 | p-Cresol | C7H8O | 0.49 | 1.59 | / |
| 5.564 | Guaiacol | C7H8O2 | 1.58 | 1.40 | 0.44 |
| 5.623 | Cyclopropyl carbinol | C4H8O | 0.85 | 0.80 | 6.24 |
| 5.812 | 3-Hydroxypyridine | C5H5NO | / | / | 5.71 |
| 6.123 | 4H-Pyran-4-one,2,3-dihydro-3,5-dihydroxy-6-methyl- | C6H8O4 | / | 2.14 | / |
| 6.811 | 2,3-Dihydrobenzofuran | C8H8O | 8.79 | 2.05 | 2.82 |
| 6.919 | 5-Hydroxymethylfurfural | C6H6O3 | / | 3.45 | / |
| 7.399 | Hydroquinone | C6H6O2 | / | / | 1.64 |
| 7.741 | Ethanone,1-(4-hydroxy-2-methylphenyl)- | C9H10O2 | / | / | 1.66 |
| 7.746 | 4-Hydroxy-3-methoxystyrene | C9H10O2 | 3.19 | 1.25 | / |
| 8.068 | 2,6-Dimethoxyphenol | C8H10O3 | 4.23 | 4.20 | 3.50 |
| 8.208 | 3-Hydroxybenzaldehyde | C7H6O2 | / | / | 1.80 |
| 8.67 | Amyl acetate | C7H14O2 | / | / | 2.65 |
| 8.948 | iso-Eugenol | C10H12O2 | 1.30 | / | / |
| 9.288 | Benzenethiol,4-(1,1-dimethylethyl)- | C10H14S | 1.23 | / | / |
| 9.347 | 1,6-Anhydro-β-d-glucopyranose | C6H10O5 | 1.68 | / | / |
| 9.641 | 2-Propanone,1-(4-hydroxy-3-methoxyphenyl)- | C10H12O3 | 0.28 | / | 2.79 |
| 9.89 | 3′,5′-Dimethoxyacetophenone | C10H12O3 | 3.37 | / | / |
| 10.661 | Syringaldehyde | C9H10O4 | 1.33 | / | 3.40 |
| 10.938 | Methoxyeugenol | C11H14O3 | 2.08 | / | 1.59 |
| 11.21 | Acetosyringone | C10H12O4 | 1.00 | / | 3.22 |
| 11.258 | Coniferol | C10H12O3 | 4.97 | / | / |
| 11.469 | Benzeneacetic acid,4-hydroxy-3,5-dimethoxy- | C10H12O5 | 0.84 | 0.87 | 10.27 |
| 13.002 | 5,6-Dimethoxyphthalaldehydic acid | C10H10O5 | 5.29 | / | / |
| Unknown | 21.28 | 12.81 | 27.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Pan, X.; Qiao, H.; Zhuang, X. Analytical Pyrolysis of Soluble Bio-Tar from Steam Pretreatment of Bamboo by Using TG–FTIR and Py–GC/MS. Materials 2024, 17, 1985. https://doi.org/10.3390/ma17091985
Feng Y, Pan X, Qiao H, Zhuang X. Analytical Pyrolysis of Soluble Bio-Tar from Steam Pretreatment of Bamboo by Using TG–FTIR and Py–GC/MS. Materials. 2024; 17(9):1985. https://doi.org/10.3390/ma17091985
Chicago/Turabian StyleFeng, Yongshun, Xin Pan, Hui Qiao, and Xiaowei Zhuang. 2024. "Analytical Pyrolysis of Soluble Bio-Tar from Steam Pretreatment of Bamboo by Using TG–FTIR and Py–GC/MS" Materials 17, no. 9: 1985. https://doi.org/10.3390/ma17091985