Degree of Impurity and Carbon Contents in the Grain Size of Mg-Al Magnesium Alloys
Abstract
:1. Introduction
2. Materials and Experiments
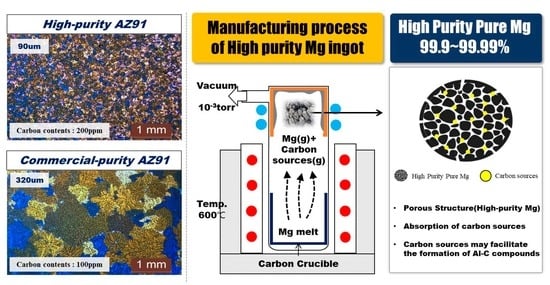
2.1. Materials and Casting Process
2.2. Measurement of Grain Size
2.3. Thermal Analysis
2.4. Manufacturing of Rapidly Solidified Ribbon Samples and Particle Analysis
2.5. Analysis of Carbon Composition and Experiment on Carbon Sources Generation
3. Results & Discussion
3.1. Grain Structure and Degree of Undercooling
3.2. Carbon Contents
3.3. Particles in the Rapidly Solidified Microstructure
3.4. Carbon Sources
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, J.; Chen, J.; Xiong, X.; Peng, X.; Chen, D.; Pan, F. Research Advances of Magnesium and Magnesium Alloys Worldwide in 2021. J. Magnes. Alloy. 2022, 10, 863–898. [Google Scholar] [CrossRef]
- Kaya, A.A. A Review on Developments in Magnesium Alloys. Front. Mater. 2020, 7, 198. [Google Scholar] [CrossRef]
- Mordike, B.L.; Ebert, T. Magnesium Properties—Applications—Potential. Mater. Sci. Eng. A 2001, 302, 37–45. [Google Scholar] [CrossRef]
- Easton, M.A.; Qian, M.; StJohn, D.H. Grain Refinement in Alloys: Novel Approaches. In Encyclopedia of Materials: Science and Technology; Elsevier: Amsterdam, The Netherlands, 2011; pp. 1–7. [Google Scholar] [CrossRef]
- Qian, M.; StJohn, D.H.; Frost, M.T. Characteristic Zirconium-Rich Coring Structures in Mg-Zr Alloys. Scr. Mater. 2002, 46, 649–654. [Google Scholar] [CrossRef]
- Lee, Y.C.; Dahle, A.K.; Stjohn, D.H. The Role of Solute in Grain Refinement of Magnesium. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2000, 31, 2895–2906. [Google Scholar] [CrossRef]
- Jung, S.S.; Son, Y.G.; Park, Y.H.; Lee, Y.C. A Study on the Grain Refining Mechanisms and Melt Superheat Treatment of Aluminum-Bearing Mg Alloys. Metals 2022, 12, 464. [Google Scholar] [CrossRef]
- Stjohn, D.H.; Easton, M.A.; Qian, M.; Taylor, J.A. Grain Refinement of Magnesium Alloys: A Review of Recent Research, Theoretical Developments, and Their Application. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2013, 44, 2935–2949. [Google Scholar] [CrossRef] [Green Version]
- Tamura, Y.; Haitani, T.; Yano, E.; Motegi, T.; Kono, N.; Sato, E. Grain Refinement of High-Purity Mg-Al Alloy Ingots and Influences of Minor Amounts of Iron and Manganese on Cast Grain Size. Mater. Trans. 2002, 43, 2784–2788. [Google Scholar] [CrossRef] [Green Version]
- Tamura, Y.; Yagi, J.; Haitani, T.; Motegi, T.; Kono, N.; Tamehiro, H.; Saito, H. Observation of Manganese-Bearing Particles in Molten AZ91 Magnesium Alloy by Rapid Solidification. Mater. Trans. 2003, 44, 552–557. [Google Scholar] [CrossRef] [Green Version]
- Cao, P.; Qian, M.; StJohn, D.H. Effect of Iron on Grain Refinement of High-Purity Mg-Al Alloys. Scr. Mater. 2004, 51, 125–129. [Google Scholar] [CrossRef]
- Cao, P.; Qian, M.; Stjohn, D.H. Native Grain Refinement of Magnesium Alloys. Scr. Mater. 2005, 53, 841–844. [Google Scholar] [CrossRef]
- Srinivasan, A.; Pillai, U.T.S.; Pai, B.C. Microstructure and Mechanical Properties of Si and Sb Added AZ91 Magnesium Alloy. Metall. Mater. Trans. A 2005, 36, 2235–2243. [Google Scholar] [CrossRef]
- Han, M.; Zhu, X.; Gao, T.; Liu, X. Revealing the Roles of Al4C3and Al8Mn5during α-Mg Nucleation in Mg-Al Based Alloys. J. Alloys Compd. 2017, 705, 14–21. [Google Scholar] [CrossRef]
- Tamura, Y.; Haitani, T.; Kono, N. Liquid Solubility of Manganese and Its Influence on Grain Size of Mg-Al Alloys. Mater. Trans. 2006, 47, 1968–1974. [Google Scholar] [CrossRef]
- Men, H.; Jiang, B.; Fan, Z. Mechanisms of Grain Refinement by Intensive Shearing of AZ91 Alloy Melt. Acta Mater. 2010, 58, 6526–6534. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.J.; Chen, X.D.; Xia, T.D.; Yu, W.Y.; Wang, X.L. Influencing Factors and Estimation of the Cooling Rate within an Amorphous Ribbon. Intermetallics 2004, 12, 1233–1237. [Google Scholar] [CrossRef]
- Neelameggham, N.R. Primary Production of Magnesium; Woodhead Publishing Limited: Soston, UK, 2013; ISBN 9780857090881. [Google Scholar]
- Hwang, D.J.; Yu, Y.H.; Lee, J.D. A Study on the Characteristics of Manufactured Mg Crown on the Calcining Conditions of Dolomite. Korean Chem. Eng. Res. 2021, 59, 611–625. [Google Scholar] [CrossRef]
- Baek, U.H.; Lee, B.D.; Lee, K.W.; Han, G.S.; Han, J.W. Study of the Thermal Reduction Behavior of Dolomite by the Pidgeon Process. J. Korean Inst. Met. Mater. 2016, 54, 104–112. [Google Scholar] [CrossRef]
- Revel, G.; Pastol, J.L.; Rouchaud, J.C.; Fromageau, R. Purification of Magnesium by Vacuum Distillation. Metall. Trans. B 1978, 9, 665–672. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, X.; Qu, T.; Lyu, F.; Du, H.; Shi, L.; Yang, B.; Dai, Y. Technical Research on Vacuum Distillation to Purify Magnesium to 99.99% Purity. Mater. Res. Express 2021, 8, 056506. [Google Scholar] [CrossRef]
- Wang, Y.C.; Tian, Y.; Qu, T.; Yang, B.; Dai, Y.N.; Sun, Y.P. Purification of Magnesium by Vacuum Distillation and Its Analysis. Mater. Sci. Forum 2014, 788, 52–57. [Google Scholar] [CrossRef]
- Tamura, Y.; Haitani, T.; Kono, N.; Motegi, T.; Sato, E. Vacuum Distillation of Magnesium. Keikinzoku/J. Jpn. Inst. Light Met. 1998, 48, 237–241. [Google Scholar] [CrossRef] [Green Version]
- Fang, D.; He, F.; Xie, J.; Xue, L. Calibration of Binding Energy Positions with C1s for XPS Results. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2020, 35, 711–718. [Google Scholar] [CrossRef]
- Subramanian, J.; Guan, K.C.; Kuma, J.; Gupta, M. Feasibility Study on Utilizing Carbon Dioxide during the Processing of Mg-Al Alloys. J. Mater. Process. Technol. 2011, 211, 1416–1422. [Google Scholar] [CrossRef]
- Liu, Y.; You, G.; Gao, F.; Long, S.; Liu, Q.; Shao, J.; Ao, S.; Li, X. Effect of Gaseous Carbon Dioxide on Grain Refinement in Mg-8Al Alloy. Mater. Sci. Technol. 2017, 33, 2173–2179. [Google Scholar] [CrossRef]










| Alloy | Al | Zn | Mn | Si | Fe | Cu | Ni | Mg |
|---|---|---|---|---|---|---|---|---|
| Commercial Purity AZ91 | 8.93 | 0.57 | 0.250 | 0.015 | 0.0022 | 0.0016 | 0.0012 | Bal. |
| High Purity AZ91 | 8.97 | 0.69 | 0.006 | 0.0015 | 0.0010 | 0.0006 | 0.0012 | Bal. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, S.-S.; Park, Y.-H.; Lee, Y.-C. Degree of Impurity and Carbon Contents in the Grain Size of Mg-Al Magnesium Alloys. Materials 2023, 16, 3069. https://doi.org/10.3390/ma16083069
Jung S-S, Park Y-H, Lee Y-C. Degree of Impurity and Carbon Contents in the Grain Size of Mg-Al Magnesium Alloys. Materials. 2023; 16(8):3069. https://doi.org/10.3390/ma16083069
Chicago/Turabian StyleJung, Sung-Su, Yong-Ho Park, and Young-Cheol Lee. 2023. "Degree of Impurity and Carbon Contents in the Grain Size of Mg-Al Magnesium Alloys" Materials 16, no. 8: 3069. https://doi.org/10.3390/ma16083069