Processing and Physicochemical Properties of Magnetite Nanoparticles Coated with Curcuma longa L. Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Turmeric Extract Preparation
2.2.2. Co-Precipitation Route from Iron Salts for Bare MNPs
2.2.3. Synthesis of MNPs Coated Turmeric Extract Base, G-M@T
2.3. Physicochemical Characterization
2.3.1. X-ray Diffraction
2.3.2. Fourier-Transform Infrared Spectroscopy
2.3.3. X-ray Photoelectron Spectroscopy
2.3.4. Photoacoustic Spectroscopy
2.3.5. Transmission Electron Microscopy
2.3.6. Superconducting Quantum Interference Device Magnetometer
2.3.7. Topography and Magnetic Domains
2.4. Biological Evaluation
2.4.1. Peripheral Blood Mononuclear Cells and HepG2 Cell Line
2.4.2. Treatments and Cell Viability using MTT and Neutral Red Uptake (NR) Assays
2.4.3. Statistical Analysis
3. Results
3.1. XRD Analysis
3.2. FT-IR Analysis

3.3. XPS Analysis
3.4. PAS Analysis
3.5. TE Coating, Particle Size, and Electron Diffraction
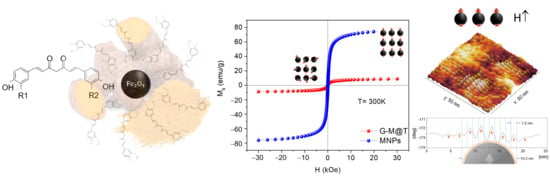
3.6. SQUID Magnetometer
3.7. Topography and Magnetic Domains
3.8. Biological Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santoyo Salazar, J.; Perez, L.; de Abril, O.; Truong Phuoc, L.; Ihiawakrim, D.; Vazquez, M.; Greneche, J.-M.; Begin-Colin, S.; Pourroy, G. Magnetic Iron Oxide Nanoparticles in 10–40 Nm Range: Composition in Terms of Magnetite/Maghemite Ratio and Effect on the Magnetic Properties. Chem. Mater. 2011, 23, 1379–1386. [Google Scholar] [CrossRef]
- Yew, Y.P.; Shameli, K.; Miyake, M.; Ahmad Khairudin, N.B.B.; Mohamad, S.E.B.; Naiki, T.; Lee, K.X. Green Biosynthesis of Superparamagnetic Magnetite Fe3O4 Nanoparticles and Biomedical Applications in Targeted Anticancer Drug Delivery System: A Review. Arab. J. Chem. 2020, 13, 2287–2308. [Google Scholar] [CrossRef]
- Liandi, A.R.; Cahyana, A.H.; Yunarti, R.T.; Wendari, T.P. Facile Synthesis of Magnetic Fe3O4@Chitosan Nanocomposite as Environmentally Green Catalyst in Multicomponent Knoevenagel-Michael Domino Reaction. Ceram. Int. 2022, 48, 20266–20274. [Google Scholar] [CrossRef]
- Thu Huong, L.T.; Nam, N.H.; Doan, D.H.; My Nhung, H.T.; Quang, B.T.; Nam, P.H.; Thong, P.Q.; Phuc, N.X.; Thu, H.P. Folate Attached, Curcumin Loaded Fe3O4 Nanoparticles: A Novel Multifunctional Drug Delivery System for Cancer Treatment. Mater. Chem. Phys. 2016, 172, 98–104. [Google Scholar] [CrossRef]
- Tao, C.; Chen, T.; Liu, H.; Su, S. Design of Biocompatible Fe3O4@MPDA Mesoporous Core-Shell Nanospheres for Drug Delivery. Microporous Mesoporous Mater. 2020, 293, 109823. [Google Scholar] [CrossRef]
- Pazouki, N.; Irani, S.; Olov, N.; Atyabi, S.M.; Bagheri-Khoulenjani, S. Fe3O4 Nanoparticles Coated with Carboxymethyl Chitosan Containing Curcumin in Combination with Hyperthermia Induced Apoptosis in Breast Cancer Cells. Prog. Biomater. 2022, 11, 43–54. [Google Scholar] [CrossRef]
- Wu, K.; Mohsin, A.; Zaman, W.Q.; Zhang, Z.; Guan, W.; Chu, M.; Zhuang, Y.; Guo, M. Urchin-like Magnetic Microspheres for Cancer Therapy through Synergistic Effect of Mechanical Force, Photothermal and Photodynamic Effects. J. Nanobiotechnol. 2022, 20, 224. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.G.M.; Martín, M.J.; Otarola, J.; Vakarelska, E.; Simeonov, V.; Lassalle, V.; Nedyalkova, M. Biomedical Applications of Iron Oxide Nanoparticles: Current Insights Progress and Perspectives. Pharmaceutics 2022, 14, 204. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Rubio, I.; Rodrigo, I.; Olazagoitia-Garmendia, A.; Arriortua, O.; Gil de Muro, I.; Garitaonandia, J.S.; Bilbao, J.R.; Fdez-Gubieda, M.L.; Plazaola, F.; Orue, I.; et al. Highly Reproducible Hyperthermia Response in Water, Agar, and Cellular Environment by Discretely PEGylated Magnetite Nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 27917–27929. [Google Scholar] [CrossRef]
- Ramnandan, D.; Mokhosi, S.; Daniels, A.; Singh, M. Chitosan, Polyethylene Glycol and Polyvinyl Alcohol Modified MgFe2O4 Ferrite Magnetic Nanoparticles in Doxorubicin Delivery: A Comparative Study In Vitro. Molecules 2021, 26, 3893. [Google Scholar] [CrossRef]
- Hu, W.; Qi, Q.; Hu, H.; Wang, C.; Zhang, Q.; Zhang, Z.; Zhao, Y.; Yu, X.; Guo, M.; Du, S.; et al. Fe3O4 Liposome for Photothermal/Chemo-Synergistic Inhibition of Metastatic Breast Tumor. Colloids Surf. A Phys. Eng. Asp. 2022, 634, 127921. [Google Scholar] [CrossRef]
- Wang, K.; Xu, X.-G.; Ma, Y.-L.; Sheng, C.-R.; Li, L.-N.; Lu, L.-Y.; Wang, J.; Wang, Y.-N.; Jiang, Y. Fe3O4@Angelica Sinensis Polysaccharide Nanoparticles as an Ultralow-Toxicity Contrast Agent for Magnetic Resonance Imaging. Rare Met. 2021, 40, 2486–2493. [Google Scholar] [CrossRef]
- Yusefi, M.; Shameli, K.; Hedayatnasab, Z.; Teow, S.-Y.; Ismail, U.N.; Azlan, C.A.; Rasit Ali, R. Green Synthesis of Fe3O4 Nanoparticles for Hyperthermia, Magnetic Resonance Imaging and 5-Fluorouracil Carrier in Potential Colorectal Cancer Treatment. Res. Chem. Intermed. 2021, 47, 1789–1808. [Google Scholar] [CrossRef]
- Zou, Y.; Huang, B.; Cao, L.; Deng, Y.; Su, J. Tailored Mesoporous Inorganic Biomaterials: Assembly, Functionalization, and Drug Delivery Engineering. Adv. Mater. 2021, 33, 2005215. [Google Scholar] [CrossRef]
- Hapuarachchige, S.; Artemov, D. Theranostic Pretargeting Drug Delivery and Imaging Platforms in Cancer Precision Medicine. Front. Oncol. 2020, 10, 1131. [Google Scholar] [CrossRef]
- Ganapathe, L.S.; Mohamed, M.A.; Mohamad Yunus, R.; Berhanuddin, D.D. Magnetite (Fe3O4) Nanoparticles in Biomedical Application: From Synthesis to Surface Functionalisation. Magnetochemistry 2020, 6, 68. [Google Scholar] [CrossRef]
- Beyene, A.M.; Moniruzzaman, M.; Karthikeyan, A.; Min, T. Curcumin Nanoformulations with Metal Oxide Nanomaterials for Biomedical Applications. Nanomaterials 2021, 11, 460. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, Q.; Wu, F.; Dai, Y.; Chen, X. Polyphenol-Containing Nanoparticles: Synthesis, Properties, and Therapeutic Delivery. Adv. Mater. 2021, 33, 2007356. [Google Scholar] [CrossRef]
- Ramirez-Nuñez, A.L.; Jimenez-Garcia, L.F.; Goya, G.F.; Sanz, B.; Santoyo-Salazar, J. In Vitro Magnetic Hyperthermia Using Polyphenol-Coated Fe3O4@γFe2O3 Nanoparticles from Cinnamomun verum and Vanilla planifolia: The Concert of Green Synthesis and Therapeutic Possibilities. Nanotechnology 2018, 29, 074001. [Google Scholar] [CrossRef] [PubMed]
- Shameli, K.; Ahmad, M.; Shabanzadeh, P.; Zamanian, A.; Sangpour, P.; Abdollahi, Y.; Mohsen, Z. Green Biosynthesis of Silver Nanoparticles Using Curcuma Longa Tuber Powder. Int. J. Nanomed. 2012, 7, 5603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hazafa, A.; Rehman, K.-U.-; Jahan, N.; Jabeen, Z. The Role of Polyphenol (Flavonoids) Compounds in the Treatment of Cancer Cells. Nutr. Cancer 2020, 72, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Geng, H.; Yu, Q.; Hao, J.; Cui, J. Polyphenol-Based Particles for Theranostics. Theranostics 2019, 9, 3170–3190. [Google Scholar] [CrossRef] [PubMed]
- Maleki Dana, P.; Sadoughi, F.; Asemi, Z.; Yousefi, B. The Role of Polyphenols in Overcoming Cancer Drug Resistance: A Comprehensive Review. Cell. Mol. Biol. Lett. 2022, 27, 1. [Google Scholar] [CrossRef] [PubMed]
- Ayati, Z.; Ramezani, M.; Amiri, M.S.; Moghadam, A.T.; Rahimi, H.; Abdollahzade, A.; Sahebkar, A.; Emami, S.A. Ethnobotany, Phytochemistry and Traditional Uses of Curcuma Spp. and Pharmacological Profile of Two Important Species (C. longa and C. zedoaria): A Review. Curr. Pharm. Des. 2019, 25, 871–935. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Noguez, M.L.; Rojas-Franco, P.; Cano-Europa, E.; Franco-Colín, M.; Hernández-Aguilar, C.; Domínguez-Pacheco, F.A.; Cruz-Orea, A.; Sánchez-Sinencio, F. Curcuma Longa Treatment Effect on Blood Samples of Rat with Hepatic Damage: A Photoacoustic Spectroscopy Application. Int. J. Thermophys. 2018, 39, 105. [Google Scholar] [CrossRef]
- Alvarado–Noguez, M.L.; Hernández-Aguilar, C.; Cruz–Orea, A.; Domínguez-Pacheco, F.A. Blood Optical Absorption of Rats with Hepatic Damage and Turmeric Treatment: Methemoglobin Analysis. J. Mol. Liq. 2019, 291, 111310. [Google Scholar] [CrossRef]
- Yang, Q.-Q.; Cheng, L.-Z.; Zhang, T.; Yaron, S.; Jiang, H.-X.; Sui, Z.-Q.; Corke, H. Phenolic Profiles, Antioxidant, and Antiproliferative Activities of Turmeric (Curcuma longa). Ind. Crops Prod. 2020, 152, 112561. [Google Scholar] [CrossRef]
- Fatima, G.; Loubna, A.; Wiame, L.; Azeddine, I. In Silico Inhibition Studies of AXL Kinase by Curcumin and Its Natural Derivatives. J. Appl. Bioinform. Comput. Biol. 2017, 6, 2. [Google Scholar] [CrossRef] [Green Version]
- Hishikawa, N.; Takahashi, Y.; Amakusa, Y.; Tanno, Y.; Tuji, Y.; Niwa, H.; Murakami, N.; Krishna, U. Effects of Turmeric on Alzheimer’s Disease with Behavioral and Psychological Symptoms of Dementia. AYU (Int. Q. J. Res. Ayurveda) 2012, 33, 499. [Google Scholar] [CrossRef] [Green Version]
- Tamvakopoulos, C.; Dimas, K.; Sofianos, Z.D.; Hatziantoniou, S.; Han, Z.; Liu, Z.L.; Wyche, J.H.; Pantazis, P. Metabolism and Anticancer Activity of the Curcumin Analogue, Dimethoxycurcumin. Clin. Cancer Res. 2007, 13, 1269–1277. [Google Scholar] [CrossRef] [Green Version]
- Ruby, A.J.; Kuttan, G.; Dinesh Babu, K.; Rajasekharan, K.N.; Kuttan, R. Anti-Tumour and Antioxidant Activity of Natural Curcuminoids. Cancer Lett. 1995, 94, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Lobo, R.; Prabhu, K.S.; Shirwaikar, A.; Shirwaikar, A. Curcuma zedoaria Rosc. (White Turmeric): A Review of Its Chemical, Pharmacological and Ethnomedicinal Properties. J. Pharm. Pharmacol. 2009, 61, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Vaz, G.R.; Clementino, A.; Bidone, J.; Villetti, M.A.; Falkembach, M.; Batista, M.; Barros, P.; Sonvico, F.; Dora, C. Curcumin and Quercetin-Loaded Nanoemulsions: Physicochemical Compatibility Study and Validation of a Simultaneous Quantification Method. Nanomaterials 2020, 10, 1650. [Google Scholar] [CrossRef]
- Nigam, S.; Kumar, A.; Thouas, G.A.; Bahadur, D.; Chen, Q. Curcumin Delivery Using Magnetic Liposomes. J. Nanopharm. Drug Deliv. 2013, 1, 365–375. [Google Scholar] [CrossRef]
- Ismail, N.I.; Othman, I.; Abas, F.H.; Lajis, N.; Naidu, R. The Curcumin Analogue, MS13 (1,5-Bis(4-Hydroxy-3- Methoxyphenyl)-1,4-Pentadiene-3-One), Inhibits Cell Proliferation and Induces Apoptosis in Primary and Metastatic Human Colon Cancer Cells. Molecules 2020, 25, 3798. [Google Scholar] [CrossRef]
- Kim, K.-C.; Baek, S.-H.; Lee, C. Curcumin-Induced Downregulation of Axl Receptor Tyrosine Kinase Inhibits Cell Proliferation and Circumvents Chemoresistance in Non-Small Lung Cancer Cells. Int. J. Oncol. 2015, 47, 2296–2303. [Google Scholar] [CrossRef] [Green Version]
- Jayaprakasha, G.K.; Jaganmohan Rao, L.; Sakariah, K.K. Antioxidant Activities of Curcumin, Demethoxycurcumin and Bisdemethoxycurcumin. Food Chem. 2006, 98, 720–724. [Google Scholar] [CrossRef]
- Hatamipour, M.; Ramezani, M.; Tabassi, S.A.S.; Johnston, T.P.; Sahebkar, A. Demethoxycurcumin: A Naturally Occurring Curcumin Analogue for Treating Non-Cancerous Diseases. J. Cell. Physiol. 2019, 234, 19320–19330. [Google Scholar] [CrossRef]
- Lee, Y.S.; Oh, S.M.; Li, Q.Q.; Kim, K.W.; Yoon, D.; Lee, M.H.; Kwon, D.Y.; Kang, O.H.; Lee, D.Y. Validation of a Quantification Method for Curcumin Derivatives and Their Hepatoprotective Effects on Nonalcoholic Fatty Liver Disease. Curr. Issues Mol. Biol. 2022, 44, 409–432. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Jiang, H.G.; Shu, Y.; Chen, Y.J.; Jin, J.; Zhu, Y.M.; Li, M.Y.; Wu, J.N.; Li, J. Bisdemethoxycurcumin Enhances the Sensitivity of Non-Small Cell Lung Cancer Cells to Icotinib via Dual Induction of Autophagy and Apoptosis. Int. J. Biol. Sci. 2020, 16, 1536–1550. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.B.; Kang, O.H.; Lee, Y.S.; Han, S.H.; Ahn, Y.S.; Cha, S.W.; Seo, Y.S.; Kong, R.; Kwon, D.Y. Hepatoprotective Effect and Synergism of Bisdemethoycurcumin against MCD Diet-Induced Nonalcoholic Fatty Liver Disease in Mice. PLoS ONE 2016, 11, e0147745. [Google Scholar] [CrossRef]
- Chainani-Wu, N. Safety and Anti-Inflammatory Activity of Curcumin: A Component of Tumeric (Curcuma longa). J. Altern. Complement. Med. 2003, 9, 161–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khosravi, A.; Hasani, A.; Rahimi, K.; Aliaghaei, A.; Pirani, M.; Azad, N.; Ramezani, F.; Tamimi, A.; Behnam, P.; Raoofi, A.; et al. Ameliorating Effects of Curcumin-Loaded Superparamagnetic Iron Oxide Nanoparticles (SPIONs) on the Mouse Testis Exposed to the Transient Hyperthermia: A Molecular and Stereological Study. Acta Histochem. 2020, 122, 151632. [Google Scholar] [CrossRef] [PubMed]
- Sundaramurthy, A. Phytosynthesized Nanomaterials—NextGen Material for Biomedical Applications. In Emerging Phytosynthesized Nanomaterials for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2023; pp. 31–64. [Google Scholar]
- Massart, R. Preparation of Aqueous Magnetic Liquids in Alkaline and Acidic Media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, K.; Hasija, V.; Sharma, V.; Sharma, S.; Raizada, P.; Singh, M.; Saini, A.K.; Hosseini-Bandegharaei, A.; Thakur, V.K. Systematic Review on Applicability of Magnetic Iron Oxides–Integrated Photocatalysts for Degradation of Organic Pollutants in Water. Mater Today Chem. 2019, 14, 100186. [Google Scholar] [CrossRef]
- Salehipour, M.; Rezaei, S.; Mosafer, J.; Pakdin-Parizi, Z.; Motaharian, A.; Mogharabi-Manzari, M. Recent Advances in Polymer-Coated Iron Oxide Nanoparticles as Magnetic Resonance Imaging Contrast Agents. J. Nanoparticle Res. 2021, 23, 48. [Google Scholar] [CrossRef]
- Aisida, S.O.; Akpa, P.A.; Ahmad, I.; Zhao, T.K.; Maaza, M.; Ezema, F.I. Bio-Inspired Encapsulation and Functionalization of Iron Oxide Nanoparticles for Biomedical Applications. Eur. Polym. J. 2020, 122, 109371. [Google Scholar] [CrossRef]
- Herrera-Becerra, R.; Zorrilla, C.; Ascencio, J.A. Production of Iron Oxide Nanoparticles by a Biosynthesis Method: An Environmentally Friendly Route. J. Phys. Chem. C 2007, 111, 16147–16153. [Google Scholar] [CrossRef]
- Awwad, A.M.; Salem, N.M. A Green and Facile Approach for Synthesis of Magnetite Nanoparticles. Nanosci. Nanotechnol. 2013, 2, 208–213. [Google Scholar] [CrossRef] [Green Version]
- Vayssières, L.; Chanéac, C.; Tronc, E.; Jolivet, J.P. Size Tailoring of Magnetite Particles Formed by Aqueous Precipitation: An Example of Thermodynamic Stability of Nanometric Oxide Particles. J. Colloid. Interface Sci. 1998, 205, 205–212. [Google Scholar] [CrossRef]
- Alvis, A.; Arrazola, G.; Martinez, W. Evaluación de La Actividad y El Potencial Antioxidante de Extractos Hidro-Alcohólicos de Cúrcuma (Cúrcuma longa). Inf. Tecnol. 2012, 23, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Kraus, W.; Nolze, G. Computer Programs Powder Cell-a Program for the Representation and Manipulation of Crystal Structures and Calculation of the Resulting X-Ray Powder Patterns. J. Appl. Crystallogr. 1996, 29, 301–303. [Google Scholar] [CrossRef]
- Radoń, A.; Drygała, A.; Hawełek, Ł.; Łukowiec, D. Structure and Optical Properties of Fe 3 O 4 Nanoparticles Synthesized by Co-Precipitation Method with Different Organic Modifiers. Mater. Charact. 2017, 131, 148–156. [Google Scholar] [CrossRef]
- Vega, L.; Ostrosky-Wegman, P.; Fortou1, T.I.; Díaz, C.; Madrid, V.; Saavedra, R. Sodium Arsenite Reduces Proliferation of Human Activated T-Cells by Inhibition of the Secretion of Interleukin-2. Immunopharmacol. Immunotoxicol. 1999, 21, 203–220. [Google Scholar] [CrossRef]
- Westerink, W.M.A.; Schoonen, W.G.E.J. Cytochrome P450 Enzyme Levels in HepG2 Cells and Cryopreserved Primary Human Hepatocytes and Their Induction in HepG2 Cells. Toxicol. Vitr. 2007, 21, 1581–1591. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Panwar, V.; Kumar, P.; Bansal, A.; Ray, S.S.; Jain, S.L. PEGylated Magnetic Nanoparticles (PEG@Fe3O4) as Cost Effective Alternative for Oxidative Cyanation of Tertiary Amines via C H Activation. Appl. Catal. A Gen. 2015, 498, 25–31. [Google Scholar] [CrossRef]
- Shi, D.; Sadat, M.E.; Dunn, A.W.; Mast, D.B. Photo-Fluorescent and Magnetic Properties of Iron Oxide Nanoparticles for Biomedical Applications. Nanoscale 2015, 7, 8209–8232. [Google Scholar] [CrossRef]
- Jahandar, M.; Zarrabi, A.; Shokrgozar, M.A.; Mousavi, H. Synthesis, Characterization and Application of Polyglycerol Coated Fe3O4 Nanoparticles as a Nano-Theranostics Agent. Mater. Res. Express 2015, 2, 125002. [Google Scholar] [CrossRef]
- Trisnawati, L.; Helmiyati, H. Cellulose-Fe3O4 Nanocomposite Based on Rice Husk as Catalyst for Synthesis of Methyl Ester from Waste Cooking Oil. IOP Conf. Ser. Mater. Sci. Eng. 2020, 763, 012012. [Google Scholar] [CrossRef]
- Shannon, M.; Lafeuille, J.L.; Frégière-Salomon, A.; Lefevre, S.; Galvin-King, P.; Haughey, S.A.; Burns, D.T.; Shen, X.; Kapil, A.; McGrath, T.F.; et al. The Detection and Determination of Adulterants in Turmeric Using Fourier-Transform Infrared (FTIR) Spectroscopy Coupled to Chemometric Analysis and Micro-FTIR Imaging. Food Control. 2022, 139, 109093. [Google Scholar] [CrossRef]
- Kavas, H.; Durmus, Z.; Baykal, A.; Aslan, A.; Bozkurt, A.; Toprak, M.S. Synthesis and Conductivity Evaluation of PVTri–Fe3O4 Nanocomposite. J. Non Cryst. Solids 2010, 356, 484–489. [Google Scholar] [CrossRef]
- Chumroenphat, T.; Somboonwatthanakul, I.; Saensouk, S.; Siriamornpun, S. Changes in Curcuminoids and Chemical Components of Turmeric (Curcuma longa L.) under Freeze-Drying and Low-Temperature Drying Methods. Food Chem. 2021, 339, 128121. [Google Scholar] [CrossRef]
- Zhao, Z.; Xie, M.; Li, Y.; Chen, A.; Li, G.; Zhang, J.; Hu, H.; Wang, X.; Li, S. Formation of Curcumin Nanoparticles via Solution-Enhanced Dispersion by Supercritical CO2. Int. J. Nanomed. 2015, 10, 3171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrera-Becerra, R.; Rius, J.L.; Zorrilla, C. Tannin Biosynthesis of Iron Oxide Nanoparticles. Appl. Phys. A 2010, 100, 453–459. [Google Scholar] [CrossRef]
- Sakaguchi, M.; Makino, M.; Ohura, T.; Yamamoto, K.; Enomoto, Y.; Takase, H. Surface modification of Fe3O4 nanoparticles with dextran via a coupling reaction between naked Fe3O4 mechano-cation and naked dextran mechano-anion: A new mechanism of covalent bond formation. Adv. Powder Technol. 2019, 30, 795–806. [Google Scholar] [CrossRef]
- Singh, A.K.; Yadav, S.; Sharma, K.; Firdaus, Z.; Aditi, P.; Neogi, K.; Bansal, M.; Gupta, M.K.; Shanker, A.; Singh, R.K.; et al. Quantum Curcumin Mediated Inhibition of Gingipains and Mixed-Biofilm of Porphyromonas gingivalis Causing Chronic Periodontitis. RSC Adv. 2018, 8, 40426–40445. [Google Scholar] [CrossRef] [Green Version]
- Michels, L.; Richter, A.; Chellappan, R.K.; Røst, H.I.; Behsen, A.; Wells, K.H.; Leal, L.; Santana, V.; Blawid, R.; da Silva, G.J.; et al. Electronic and Structural Properties of the Natural Dyes Curcumin, Bixin and Indigo. RSC Adv. 2021, 11, 14169–14177. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Jin, C.; Klencsár, Z.; Ganeshraja, A.; Wang, J. Cobalt-Iron Oxide, Alloy and Nitride: Synthesis, Characterization and Application in Catalytic Peroxymonosulfate Activation for Orange II Degradation. Catalysts 2017, 7, 138. [Google Scholar] [CrossRef] [Green Version]
- Song, K.; Lee, Y.; Jo, M.R.; Nam, K.M.; Kang, Y.-M. Comprehensive Design of Carbon-Encapsulated Fe3O4 Nanocrystals and Their Lithium Storage Properties. Nanotechnology 2012, 23, 505401. [Google Scholar] [CrossRef]
- Fujii, T.; de Groot, F.M.F.; Sawatzky, G.A.; Voogt, F.C.; Hibma, T.; Okada, K. In situ XPS analysis of various iron oxide films grown by NO2-assisted molecular-beam epitaxy. Phys. Rev. B 1999, 59, 3195–3202. [Google Scholar] [CrossRef] [Green Version]
- Granada-Ramirez, D.A.; Arias-Cerón, J.S.; Pérez-González, M.; Luna-Arias, J.P.; Cruz-Orea, A.; Rodríguez-Fragoso, P.; Herrera-Pérez, J.L.; Gómez-Herrera, M.L.; Tomás, S.A.; Vázquez-Hernández, F.; et al. Chemical Synthesis and Optical, Structural, and Surface Characterization of InP-In2O3 Quantum Dots. Appl. Surf. Sci. 2020, 530, 147294. [Google Scholar] [CrossRef] [PubMed]
- Granada-Ramirez, D.A.; Arias-Cerón, J.S.; Gómez-Herrera, M.L.; Luna-Arias, J.P.; Pérez-González, M.; Tomás, S.A.; Rodríguez-Fragoso, P.; Mendoza-Alvarez, J.G. Effect of the Indium Myristate Precursor Concentration on the Structural, Optical, Chemical Surface, and Electronic Properties of InP Quantum Dots Passivated with ZnS. J. Mater. Sci. Mater. Electron. 2019, 30, 4885–4894. [Google Scholar] [CrossRef]
- Aronniemi, M.; Lahtinen, J.; Hautojärvi, P. Characterization of Iron Oxide Thin Films. Surf. Interface Anal. 2004, 36, 1004–1006. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of Multiplet Splitting of Fe 2p XPS Spectra and Bonding in Iron Compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Khalil, M.; Ismail, M.I.M.; El Ghandoor, H.; Zidan, H.M.; Khalil, M.M.H. Synthesis and Some Physical Properties of Magnetite (Fe3O4) Nanoparticles. Int. J. Electrochem. Sci. 2012, 7, 5734–5745. [Google Scholar]
- Ding, Y.; Liu, F.; Jiang, Q.; Du, B.; Sun, H. 12-Hydrothermal Synthesis and Characterization of Fe3O4 Nanorods. J. Inorg. Organomet. Polym. Mater. 2013, 23, 379–384. [Google Scholar] [CrossRef]
- Lu, A.-H.; Salabas, E.L.; Schüth, F. Magnetic Nanoparticles: Synthesis, Protection, Functionalization, and Application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef]
- de Biasi, E.; Ramos, C.A.; Zysler, R.D.; Romero, H. Large Surface Magnetic Contribution in Amorphous Ferromagnetic Nanoparticles. Phys. Rev. B 2002, 65, 144416. [Google Scholar] [CrossRef]
- Eguía-Eguía, S.I.; Gildo-Ortiz, L.; Pérez-González, M.; Tomas, S.A.; Arenas-Alatorre, J.A.; Santoyo-Salazar, J. Magnetic Domains Orientation in (Fe3O4/3-Fe2O3) Nanoparticles Coated by Gadolinium-Diethylenetriaminepentaacetic Acid (Gd3+-DTPA). Nano. Express. 2021, 2, 020019. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Kamal Abdel-Aziz, A.; Abdelfatah, S.; Abdellatif, M.; Abdoli, A.; Abel, S.; Abeliovich, H.; Abildgaard, M.H.; Princely Abudu, Y.; Acevedo-Arozena, A.; et al. Guidelines for the Use and Interpretation of Assays for Monitoring Autophagy. Autophagy 2021, 17, 1–382. [Google Scholar] [CrossRef]
- Abuammar, H.; Bhattacharjee, A.; Simon-Vecsei, Z.; Blastyák, A.; Csordás, G.; Páli, T.; Juhász, G. Ion Channels and Pumps in Autophagy: A Reciprocal Relationship. Cells 2021, 10, 3537. [Google Scholar] [CrossRef]
- Hami, Z. Coating Iron Oxide Nanoparticles with Chitosan for Targeted Delivery of Nanocurcumin. Ann. Mil. Health Sci. Res. 2020, 18, e103657. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, T.; Hayes, P. Analysis of XPS Spectra of Fe2+ and Fe3+ Ions in Oxide Materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Ribeiro, V.G.P.; Barreto, A.C.H.; Denardin, J.C.; Mele, G.; Carbone, L.; Mazzetto, S.E.; Sousa, E.M.B.; Fechine, P.B.A. Magnetic Nanoparticles Coated with Anacardic Acid Derived from Cashew Nut Shell Liquid. J. Mater. Sci. 2013, 48, 7875–7882. [Google Scholar] [CrossRef]
- Bhandari, R.; Gupta, P.; Dziubla, T.; Hilt, J.Z. Single Step Synthesis, Characterization and Applications of Curcumin Functionalized Iron Oxide Magnetic Nanoparticles. Mater. Sci. Eng. C 2016, 67, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Tavares, M.I.B.; Sergio, P.; Cruz Da Silva, R.; Rangel, S.; Da Silva, C. Evaluation of Commercial and Fresh-Picked Origanum Vulgare after Dehydration Crystal Engineering of New Antidepressants Formulations View Project Polymer Blends View Project. Int. Res. J. Mater. Sci. Appl. 2017, 1. [Google Scholar] [CrossRef]
- Passaglia, E.; Campanella, B.; Coiai, S.; Cicogna, F.; Carducci, A.; Verani, M.; Federigi, I.; Casini, B.; Tuvo, B.; Bramanti, E. Agri-Food Extracts Effectiveness in Improving Antibacterial and Antiviral Properties of Face Masks: A Proof-of-Concept Study. ChemistrySelect 2021, 6, 2288–2297. [Google Scholar] [CrossRef] [PubMed]
- Nadagouda, M.N.; Iyanna, N.; Lalley, J.; Han, C.; Dionysiou, D.D.; Varma, R.S. Synthesis of Silver and Gold Nanoparticles Using Antioxidants from Blackberry, Blueberry, Pomegranate, and Turmeric Extracts. ACS Sustain. Chem. Eng. 2014, 2, 1717–1723. [Google Scholar] [CrossRef]
- Holmes, F.D.F.; Mould, A.P.; Chapman, J.A. Morphology of Sheet-like Assemblies of PN-Collagen, PC-Collagen and Procollagen Studied by Scanning Transmission Electron Microscopy Mass Measurements. J. Mol. Biol. 1991, 220, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Kittelson, D.B.; McMurry, P.H. Structural Properties of Diesel Exhaust Particles Measured by Transmission Electron Microscopy (TEM): Relationships to Particle Mass and Mobility. Aerosol. Sci. Technol. 2004, 38, 881–889. [Google Scholar] [CrossRef]
- Loferer-Krossbacher, M.; Klima, J.; Psenner, R. Determination of Bacterial Cell Dry Mass by Transmission Electron Microscopy and Densitometric Image Analysis. Appl. Environ. Microbiol. 1998, 64, 688–694. [Google Scholar] [CrossRef] [Green Version]
- De Ramos, J.D.; Santiago, M.R.; Barreto, R.P.; Mae Talaro, N.G.; Cullat, J.R. Determination of the Physical and Mechanical Properties of Turmeric (Curcuma Longa L.). Issue Philipp. J. Agric. Biosyst. Eng. 2021, 17, 27–38. [Google Scholar]
- Moore, T.L.; Rodriguez-Lorenzo, L.; Hirsch, V.; Balog, S.; Urban, D.; Jud, C.; Rothen-Rutishauser, B.; Lattuada, M.; Petri-Fink, A. Nanoparticle Colloidal Stability in Cell Culture Media and Impact on Cellular Interactions. Chem. Soc. Rev. 2015, 44, 6287–6305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, P.K.; Mishra, H.; Ekielski, A.; Talegaonkar, S.; Vaidya, B. Zinc Oxide Nanoparticles: A Promising Nanomaterial for Biomedical Applications. Drug Discov. Today 2017, 22, 1825–1834. [Google Scholar] [CrossRef]
- Kitture, R.; Ghosh, S.; Kulkarni, P.; Liu, X.L.; Maity, D.; Patil, S.I.; Jun, D.; Dushing, Y.; Laware, S.L.; Chopade, B.A.; et al. Fe3O4-Citrate-Curcumin: Promising Conjugates for Superoxide Scavenging, Tumor Suppression and Cancer Hyperthermia. J. Appl. Phys. 2012, 111, 064702. [Google Scholar] [CrossRef]
- Dutta, B.; Shetake, N.G.; Barick, B.K.; Barick, K.C.; Pandey, B.N.; Priyadarsini, K.I.; Hassan, P.A. PH Sensitive Surfactant-Stabilized Fe3O4 Magnetic Nanocarriers for Dual Drug Delivery. Colloids Surf. B Biointerfaces 2018, 162, 163–171. [Google Scholar] [CrossRef]
- Morais, P.C.; Qu, F. A Quantum Dot Model for the Surface Charge Density in Ferrite-Based Ionic Magnetic Fluids. J. Magn. Magn. Mater. 2002, 252, 117–119. [Google Scholar] [CrossRef]
- Sorasitthiyanukarn, F.N.; Muangnoi, C.; Thaweesest, W.; Ratnatilaka Na Bhuket, P.; Jantaratana, P.; Rojsitthisak, P.; Rojsitthisak, P. Polyethylene Glycol-Chitosan Oligosaccharide-Coated Superparamagnetic Iron Oxide Nanoparticles: A Novel Drug Delivery System for Curcumin Diglutaric Acid. Biomolecules 2020, 10, 73. [Google Scholar] [CrossRef] [Green Version]
- Hiremath, C.G.; Kariduraganavar, M.Y.; Hiremath, M.B. Synergistic Delivery of 5-Fluorouracil and Curcumin Using Human Serum Albumin-Coated Iron Oxide Nanoparticles by Folic Acid Targeting. Prog. Biomater. 2018, 7, 297–306. [Google Scholar] [CrossRef] [Green Version]
- Saikia, C.; Das, M.K.; Ramteke, A.; Maji, T.K. Evaluation of Folic Acid Tagged Aminated Starch/ZnO Coated Iron Oxide Nanoparticles as Targeted Curcumin Delivery System. Carbohydr. Polym. 2017, 157, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Sugumaran, A.; Sadhasivam, J.; Gawas, P.; Nutalapati, V.; Pandian, R.; Kumar Perumal, S. Curcumin Conjugated Dextran Coated Fe3O4 Nanoparticles: Cytotoxic Effect on Lung Cancer Cell Line A549. Mater. Sci. Eng. B Solid. State Mater. Adv. Technol. 2022, 286, 116047. [Google Scholar] [CrossRef]
- Shi, R.; Ow, H.; Cox, J.R.; Kmetz, A.A.; Chen, H. Optimizing Colloidal Stability and Transport of Polysaccharide-Coated Magnetic Nanoparticles for Reservoir Management: Effects of Ion Specificity. Front. Nanotechnol. 2022, 4, 864644. [Google Scholar] [CrossRef]
- Park, J.Y.; Choi, E.S.; Baek, M.J.; Lee, G.H. Colloidal Stability of Amino Acid Coated Magnetite Nanoparticles in Physiological Fluid. Mater. Lett. 2009, 63, 379–381. [Google Scholar] [CrossRef]
- Nnadozie, E.C.; Ajibade, P.A. Green Synthesis and Characterization of Magnetite (Fe3O4) Nanoparticles Using Chromolaena odorata Root Extract for Smart Nanocomposite. Mater. Lett. 2020, 263, 127145. [Google Scholar] [CrossRef]
- Giannakis, S. A Review of the Concepts, Recent Advances and Niche Applications of the (Photo) Fenton Process, beyond Water/Wastewater Treatment: Surface Functionalization, Biomass Treatment, Combatting Cancer and Other Medical Uses. Appl. Catal. B 2019, 248, 309–319. [Google Scholar] [CrossRef]
- Ramezani Farani, M.; Azarian, M.; Heydari Sheikh Hossein, H.; Abdolvahabi, Z.; Mohammadi Abgarmi, Z.; Moradi, A.; Mousavi, S.M.; Ashrafizadeh, M.; Makvandi, P.; Saeb, M.R.; et al. Folic Acid-Adorned Curcumin-Loaded Iron Oxide Nanoparticles for Cervical Cancer. ACS Appl. Bio Mater. 2022, 5, 1305–1318. [Google Scholar] [CrossRef]
- Nosrati, H.; Sefidi, N.; Sharafi, A.; Danafar, H.; Kheiri Manjili, H. Bovine Serum Albumin (BSA) Coated Iron Oxide Magnetic Nanoparticles as Biocompatible Carriers for Curcumin-Anticancer Drug. Bioorg. Chem. 2018, 76, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.K.; Yao, X.; Jana, D.; Li, M.; Zhao, Y.; Luo, Z. Efficient Production of Reactive Oxygen Species from Fe3O4/ZnPC Coloaded Nanoreactor for Cancer Therapeutics In Vivo. Small Struct. 2020, 1, 2000065. [Google Scholar] [CrossRef]
- Liang, H.; Guo, J.; Shi, Y.; Zhao, G.; Sun, S.; Sun, X. Porous Yolk-Shell Fe/Fe3O4 Nanoparticles with Controlled Exposure of Highly Active Fe(0) for Cancer Therapy. Biomaterials 2021, 268, 120530. [Google Scholar] [CrossRef]
- Ahmadian-Fard-Fini, S.; Salavati-Niasari, M.; Ghanbari, D. Hydrothermal Green Synthesis of Magnetic Fe3O4-Carbon Dots by Lemon and Grape Fruit Extracts and as a Photoluminescence Sensor for Detecting of E. Coli Bacteria. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 203, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Akaberi, M.; Sahebkar, A.; Emami, S.A. Turmeric and Curcumin: From Traditional to Modern Medicine. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2021; Volume 1291, pp. 15–39. [Google Scholar]
- Yan, F.; Li, J.; Fu, R.; Lu, Z.; Yang, W. Facile Preparation of Superparamagnetic Fe3O4/Poly(St-co-MPS)/SiO2 Composite Particles with High Magnetization by Introduction of Silanol Groups. J. Nanosci. Nanotechnol. 2009, 9, 5874–5879. [Google Scholar] [CrossRef] [PubMed]
- Rebodos, R.L.; Vikesland, P.J. Effects of Oxidation on the Magnetization of Nanoparticulate Magnetite. Langmuir 2010, 26, 16745–16753. [Google Scholar] [CrossRef] [PubMed]
- Arévalo-Cid, P.; Isasi, J.; Caballero, A.C.; Martín-Hernández, F.; González-Rubio, R. Effects of Shell-Thickness on the Powder Morphology, Magnetic Behavior and Stability of the Chitosan-Coated Fe3O4 Nanoparticles. Boletín Soc. Española Cerámica Vidr. 2022, 61, 300–312. [Google Scholar] [CrossRef]









| Sample | Lattice Parameter, a (Å) | FWHM 1 | <d> (nm) |
|---|---|---|---|
| MNPs | 8.357 | 0.853 | 13.57 |
| G-M@T | 8.344 | 1.211 | 11.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarado-Noguez, M.L.; Matías-Reyes, A.E.; Pérez-González, M.; Tomás, S.A.; Hernández-Aguilar, C.; Domínguez-Pacheco, F.A.; Arenas-Alatorre, J.A.; Cruz-Orea, A.; Carbajal-Tinoco, M.D.; Galot-Linaldi, J.; et al. Processing and Physicochemical Properties of Magnetite Nanoparticles Coated with Curcuma longa L. Extract. Materials 2023, 16, 3020. https://doi.org/10.3390/ma16083020
Alvarado-Noguez ML, Matías-Reyes AE, Pérez-González M, Tomás SA, Hernández-Aguilar C, Domínguez-Pacheco FA, Arenas-Alatorre JA, Cruz-Orea A, Carbajal-Tinoco MD, Galot-Linaldi J, et al. Processing and Physicochemical Properties of Magnetite Nanoparticles Coated with Curcuma longa L. Extract. Materials. 2023; 16(8):3020. https://doi.org/10.3390/ma16083020
Chicago/Turabian StyleAlvarado-Noguez, Margarita L., Ana E. Matías-Reyes, Mario Pérez-González, Sergio A. Tomás, Claudia Hernández-Aguilar, Flavio A. Domínguez-Pacheco, Jesús A. Arenas-Alatorre, Alfredo Cruz-Orea, Mauricio D. Carbajal-Tinoco, Jairo Galot-Linaldi, and et al. 2023. "Processing and Physicochemical Properties of Magnetite Nanoparticles Coated with Curcuma longa L. Extract" Materials 16, no. 8: 3020. https://doi.org/10.3390/ma16083020