Preparation and Hydration Properties of Steel Slag-Based Composite Cementitious Materials with High Strength
Abstract
:1. Introduction
2. Experimental
2.1. Raw Materials
2.2. Preparation and Curing Procedures
2.3. Test Methods
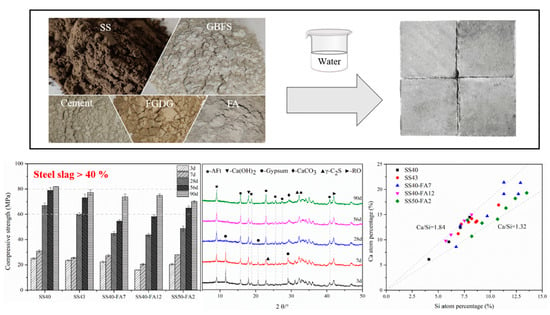
3. Results
3.1. Hydration Heat Evolution and Setting Time
3.2. Compressive Strength
3.3. Hydration Products
3.4. Length Variation
3.5. Pore Parameters
3.6. Micromorphology
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shi, C. Characteristics and cementitious properties of ladle slag fines from steel production. Cem. Concr. Res. 2002, 32, 459–462. [Google Scholar] [CrossRef]
- Lee, J.-I.; Choi, S.-J. Effect of replacement ratio of ferronickel slag aggregate on characteristics of cementitious mortars at different curing temperatures. Case Stud. Constr. Mat. 2023, 18, e01882. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, X.; Wang, S.; Liu, Z.; Liu, L.; Xu, B. Evaluating Cement Treated Aggregate Base Containing Steel Slag: Mechanical Properties, Volume Stability and Environmental Impacts. Materials 2022, 15, 8277. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xie, J.; Zhao, J.; Zuo, K. Utilization of unprocessed steel slag as fine aggregate in normal- and high-strength concrete. Constr. Build. Mater. 2019, 204, 41–49. [Google Scholar] [CrossRef]
- Han, X.; Feng, J.; Shao, Y.; Hong, R. Influence of a steel slag powder-ground fly ash composite supplementary cementitious material on the chloride and sulphate resistance of mass concrete. Powder Technol. 2020, 370, 176–183. [Google Scholar] [CrossRef]
- Zhao, Q.; Pang, L.; Wang, D. Adverse Effects of Using Metallurgical Slags as Supplementary Cementitious Materials and Aggregate: A Review. Materials 2022, 15, 3803. [Google Scholar] [CrossRef]
- Gencel, O.; Karadag, O.; Oren, O.H.; Bilir, T. Steel slag and its applications in cement and concrete technology: A review. Constr. Build. Mater. 2021, 283, 122783. [Google Scholar] [CrossRef]
- Adegoloye, G.; Beaucour, A.L.; Ortola, S.; Noumowé, A. Concretes made of EAF slag and AOD slag aggregates from stainless steel process: Mechanical properties and durability. Constr. Build. Mater. 2015, 76, 313–321. [Google Scholar] [CrossRef]
- Maslehuddin, M.; Sharif, A.M.; Shameem, M.; Ibrahim, M.; Barry, M.S. Comparison of properties of steel slag and crushed limestone aggregate concretes. Constr. Build. Mater. 2003, 17, 105–112. [Google Scholar] [CrossRef]
- Manso, J.M.; Polanco, J.A.; Losañez, M.; González, J.J. Durability of concrete made with EAF slag as aggregate. Cem. Concr. Compos. 2006, 28, 528–534. [Google Scholar] [CrossRef]
- Qasrawi, H.; Shalabi, F.; Asi, I. Use of low CaO unprocessed steel slag in concrete as fine aggregate. Constr. Build. Mater. 2009, 23, 1118–1125. [Google Scholar] [CrossRef]
- Zhu, H.; Ma, M.; He, X.; Zheng, Z.; Su, Y.; Yang, J.; Zhao, H. Effect of wet-grinding steel slag on the properties of Portland cement: An activated method and rheology analysis. Constr. Build. Mater. 2021, 286, 122823. [Google Scholar] [CrossRef]
- Kourounis, S.; Tsivilis, S.; Tsakiridis, P.; Papadimitriou, G.; Tsibouki, Z. Properties and hydration of blended cements with steelmaking slag. Cem. Concr. Res. 2007, 37, 815–822. [Google Scholar] [CrossRef]
- Rosales, J.; Cabrera, M.; Agrela, F. Effect of stainless steel slag waste as a replacement for cement in mortars. Mechanical and statistical study. Constr. Build. Mater. 2017, 142, 444–458. [Google Scholar] [CrossRef]
- Pang, L.; Liao, S.; Wang, D.; An, M. Influence of steel slag fineness on the hydration of cement-steel slag composite pastes. J. Build. Eng. 2022, 57, 104866. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Hou, G.; Yan, P. Preparation of sustainable and green cement-based composite binders with high-volume steel slag powder and ultrafine blast furnace slag powder. J. Clean. Prod. 2021, 289, 125133. [Google Scholar] [CrossRef]
- Hao, X.; Liu, X.; Zhang, Z.; Zhang, W.; Lu, Y.; Wang, Y.; Yang, T. In-depth insight into the cementitious synergistic effect of steel slag and red mud on the properties of composite cementitious materials. J. Build. Eng. 2022, 52, 104449. [Google Scholar] [CrossRef]
- He, W.; Zhao, J.; Yang, G.; Liu, Y. Investigation on the Role of Steel Slag Powder in Blended Cement Based on Quartz Powder as Reference. Adv. in Civ. Eng. 2021, 2021, 1–15. [Google Scholar] [CrossRef]
- Niu, F.; An, Y.; Zhang, J.; Chen, W.; He, S. Synergistic Excitation Mechanism of CaO-SiO2-Al2O3-SO3 Quaternary Active Cementitious System. Front. Mater. 2021, 8, 514. [Google Scholar] [CrossRef]
- Cui, X.; Ni, W.; Ren, C. Hydration Mechanism of All SolidWaste Cementitious Materials Based on Steel Slag and Blast Furnace Slag. Chin. J. Mater. Res. 2017, 31, 687–694. [Google Scholar] [CrossRef]
- Lizarazo-Marriaga, J.; Claisse, P.; Ganjian, E. Effect of Steel Slag and Portland Cement in the Rate of Hydration and Strength of Blast Furnace Slag Pastes. J. Mater. Civ. Eng. 2011, 23, 153–160. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, P.; Mi, G. Effect of blended steel slag–GBFS mineral admixture on hydration and strength of cement. Constr. Build. Mater. 2012, 35, 8–14. [Google Scholar] [CrossRef]
- Duan, S.; Liao, H.; Cheng, F.; Song, H.; Yang, H. Investigation into the synergistic effects in hydrated gelling systems containing fly ash, desulfurization gypsum and steel slag. Constr. Build. Mater. 2018, 187, 1113–1120. [Google Scholar] [CrossRef]
- Xu, C.; Ni, W.; Li, K.; Zhang, S.; Xu, D. Activation mechanisms of three types of industrial by-product gypsums on steel slag–granulated blast furnace slag-based binders. Constr. Build. Mater. 2021, 288, 123111. [Google Scholar] [CrossRef]
- Mason, B. The constitution of some open-heart slag. J. Iron Steel Inst. 1994, 11, 69–80. [Google Scholar]
- Li, Z.; Zhang, J.; Li, S.; Lin, C.; Gao, Y.; Liu, C. Feasibility of preparing red mud-based cementitious materials: Synergistic utilization of industrial solid waste, waste heat, and tail gas. J. Clean. Prod. 2021, 285, 124896. [Google Scholar] [CrossRef]
- Chen, L.; Wang, H.; Zheng, K.; Zhou, J.; He, F.; Yuan, Q. The mechanism of basic oxygen furnace steel slag retarding early-age hydration of Portland cement and mitigating approach towards higher utilization rate. J. Clean. Prod. 2022, 362, 132493. [Google Scholar] [CrossRef]
- Zhuang, S.; Wang, Q. Inhibition mechanisms of steel slag on the early-age hydration of cement. Cem. Concr. Res. 2021, 140, 106283. [Google Scholar] [CrossRef]
- Wu, M.; Sui, S.; Zhang, Y.; Jia, Y.; She, W.; Liu, Z.; Yang, Y. Analyzing the filler and activity effect of fly ash and slag on the early hydration of blended cement based on calorimetric test. Constr. Build. Mater. 2021, 276, 122201. [Google Scholar] [CrossRef]
- Federation, C.B.M. Test Methods for Water Requirement of Normal Consistency, Setting Time and Soundness of the Portland Cement; Peoples R China; China Standard Press: Beijing, China, 2011. (In Chinese) [Google Scholar]
- Nedunuri, S.S.S.A.; Sertse, S.G.; Muhammad, S. Microstructural study of Portland cement partially replaced with fly ash, ground granulated blast furnace slag and silica fume as determined by pozzolanic activity. Constr. Build. Mater. 2020, 238, 117561. [Google Scholar] [CrossRef]
- Moon, G.D.; Oh, S.; Choi, Y.C. Effects of the physicochemical properties of fly ash on the compressive strength of high-volume fly ash mortar. Constr. Build. Mater. 2016, 124, 1072–1080. [Google Scholar] [CrossRef]
- Fu, X.; Hou, W.; Yang, C.; Li, D.; Wu, X. Studies on high-strength slag and fly ash compound cement. Cem. Concr. Res. 2000, 30, 1239–1243. [Google Scholar] [CrossRef]
- Sun, Z.; Zhou, J.; Qi, Q.; Li, H.; Zhang, N.; Mu, R. Influence of Fly Ash on Mechanical Properties and Hydration of Calcium Sulfoaluminate-Activated Supersulfated Cement. Materials 2020, 13, 2514. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Ni, K.; Li, J. Properties of mortars made by uncalcined FGD gypsum-fly ash-ground granulated blast furnace slag composite binder. Waste Manag. 2012, 32, 1468–1472. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, Y.; Gao, Z. Use of steel slag as a granular material: Volume expansion prediction and usability criteria. J. Hazard. Mater. 2010, 184, 555–560. [Google Scholar] [CrossRef]
- Chen, X.; Gao, J.; Yan, Y.; Liu, Y. Investigation of expansion properties of cement paste with circulating fluidized bed fly ash. Constr. Build. Mater. 2017, 157, 1154–1162. [Google Scholar] [CrossRef]
- Dhandapani, Y.; Santhanam, M. Assessment of pore structure evolution in the limestone calcined clay cementitious system and its implications for performance. Cem. Concr. Compos. 2017, 84, 36–47. [Google Scholar] [CrossRef]
- Chen, X.; Wu, S.; Zhou, J. Influence of porosity on compressive and tensile strength of cement mortar. Constr. Build. Mater. 2013, 40, 869–874. [Google Scholar] [CrossRef]
- Choi, Y.C.; Kim, J.; Choi, S. Mercury intrusion porosimetry characterization of micropore structures of high-strength cement pastes incorporating high volume ground granulated blast-furnace slag. Constr. Build. Mater. 2017, 137, 96–103. [Google Scholar] [CrossRef]
- Saleh, F.K.; Teodoriu, C.; Sondergeld, C.H. Investigation of the effect of cement mixing energy on cement strength and porosity using NMR and UPV methods. J. Nat. Gas Sci. Eng. 2019, 70, 102972. [Google Scholar] [CrossRef]
- Qiang, W.; Mengxiao, S.; Jun, Y. Influence of classified steel slag with particle sizes smaller than 20 μm on the properties of cement and concrete. Constr. Build. Mater. 2016, 123, 601–610. [Google Scholar] [CrossRef]
- Li, Y.; Qiao, C.; Ni, W. Green concrete with ground granulated blast-furnace slag activated by desulfurization gypsum and electric arc furnace reducing slag. J. Clean. Prod. 2020, 269, 122212. [Google Scholar] [CrossRef]
- Richardson, I. The nature of C-S-H in hardened cements. Cem. Concr. Res. 1999, 29, 1131–1147. [Google Scholar] [CrossRef]
- He, Y.; Lu, L.; Struble, L.J.; Rapp, J.L.; Mondal, P.; Hu, S. Effect of calcium–silicon ratio on microstructure and nanostructure of calcium silicate hydrate synthesized by reaction of fumed silica and calcium oxide at room temperature. Mater. Struct. 2013, 47, 311–322. [Google Scholar] [CrossRef]
- Yu, P.; Kirkpatrick, R.J.; Poe, B.; McMillan, P.F.; Cong, X. Structure of Calcium Silicate Hydrate (C-S-H): Near-, Mid-, and Far-Infrared Spectroscopy. J. Am. Ceram. Soc. 1999, 82, 742–748. [Google Scholar] [CrossRef]
- Barbhuiya, S.; Mukherjee, S.; Nikraz, H. Effects of nano-Al2O3 on early-age microstructural properties of cement paste. Constr. Build. Mater. 2014, 52, 189–193. [Google Scholar] [CrossRef]
- Qomi, M.J.A.; Ulm, F.-J.; Pellenq, R.J.-M. Evidence on the Dual Nature of Aluminum in the Calcium-Silicate-Hydrates Based on Atomistic Simulations. J. Am. Ceram. Soc. 2012, 95, 1128–1137. [Google Scholar] [CrossRef]













| Materials | CaO | SiO2 | Al2O3 | SO3 | MgO | Fe2O3 | Na2O | K2O | MnO | P2O5 | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SS | 36.46 | 16.64 | 5.71 | 0.24 | 7.42 | 21.03 | 0.29 | 0.09 | 6.06 | 2.14 | 1.06 |
| GBFS | 40.38 | 30.42 | 16.74 | 1.34 | 7.56 | 1.24 | - | 0.43 | 0.23 | 0.14 | - |
| PC | 62.13 | 21.75 | 5.21 | 1.97 | 2.09 | 2.91 | - | 0.63 | 0.05 | 0.16 | 2.40 |
| FGDG | 32.06 | 1.65 | 0.80 | 42.46 | 0.70 | 0.20 | - | 0.08 | 0.02 | 0.02 | 21.59 |
| FA | 7.95 | 46.41 | 31.18 | 1.91 | 1.30 | 4.97 | 0.60 | 0.86 | 0.08 | 0.60 | 2.40 |
| Binders | SS | FGDG | PC | FA | GBFS |
|---|---|---|---|---|---|
| SS40 | 40 | 13 | 15 | 0 | 32 |
| SS43 | 43 | 13 | 15 | 0 | 29 |
| SS40-FA7 | 40 | 13 | 15 | 7 | 25 |
| SS40-FA12 | 40 | 13 | 15 | 12 | 20 |
| SS50-FA2 | 50 | 13 | 15 | 2 | 20 |
| Groups | Average Pore Diameter (nm) | Median Pore Diameter (Volume) (nm) | Porosity (%) |
|---|---|---|---|
| SS40 | 19.07 | 27.77 | 23.61 |
| SS43 | 23.10 | 34.35 | 20.91 |
| SS40-FA7 | 20.60 | 31.00 | 22.84 |
| SS40-FA12 | 19.19 | 28.91 | 23.37 |
| SS50-FA2 | 18.35 | 27.29 | 30.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Ma, Y.; Wang, J.; Shen, X. Preparation and Hydration Properties of Steel Slag-Based Composite Cementitious Materials with High Strength. Materials 2023, 16, 2764. https://doi.org/10.3390/ma16072764
Xu Z, Ma Y, Wang J, Shen X. Preparation and Hydration Properties of Steel Slag-Based Composite Cementitious Materials with High Strength. Materials. 2023; 16(7):2764. https://doi.org/10.3390/ma16072764
Chicago/Turabian StyleXu, Zhiming, Ying Ma, Jiahao Wang, and Xiaodong Shen. 2023. "Preparation and Hydration Properties of Steel Slag-Based Composite Cementitious Materials with High Strength" Materials 16, no. 7: 2764. https://doi.org/10.3390/ma16072764