Hierarchical Biobased Macroporous/Mesoporous Carbon: Fabrication, Characterization and Electrochemical/Ion Exchange Properties
Abstract
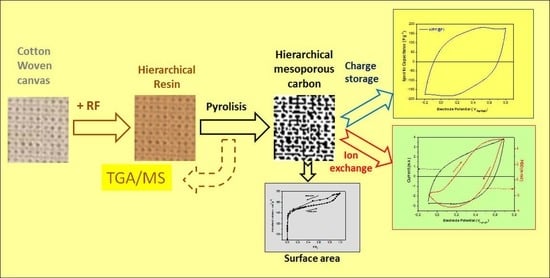
:1. Introduction
2. Materials and Methods
2.1. Resin Synthesis
2.2. Carbonization
2.3. Measurement of Surface Area
2.4. Scanning Electron Microscopy
2.5. Thermal Analysis during Carbonization
2.6. Measurement of Mechanical Properties
2.7. Electrochemical Properties
2.7.1. Cyclic Voltammetry (CV) and Electrochemical Impedance Spectroscopy(EIS)
2.7.2. Chronoamperometry (CA)
2.7.3. Probe Beam Deflection
3. Results and Discussion
3.1. Fabrication of RF Carbon Supported on Cellulosic Woven Fabric
3.2. Mechanical Properties
3.3. Morphology and Textural Properties
3.3.1. Morphology
3.3.2. Textural Properties
3.3.3. Carbonization Process
3.3.4. Electrochemical Properties
3.3.5. Potential Driven Ion Exchange
Acid Media
Neutral Media
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jiang, L.; Sheng, L.; Fan, Z. Biomass-derived carbon materials with structural diversities and their applications in energy storage. Sci. China Mater. 2018, 61, 133–158. [Google Scholar] [CrossRef] [Green Version]
- Hyun, J.C.; Kwak, J.H.; Lee, M.E.; Choi, J.; Kim, J.; Kim, S.-S.; Yun, Y.S. Intensification of Pseudocapacitance by Nanopore Engineering on Waste-Bamboo-Derived Carbon as a Positive Electrode for Lithium-Ion Batteries. Materials 2019, 12, 2733. [Google Scholar] [CrossRef] [Green Version]
- Olabi, A.G.; Abbas, Q.; Abdelkareem, M.A.; Alami, A.H.; Mirzaeian, M.; Sayed, E.T. Carbon-Based Materials for Supercapacitors: Recent Progress, Challenges and Barriers. Batteries 2023, 9, 19. [Google Scholar] [CrossRef]
- Sharma, K.; Shafi, P.M. An overview, methods of synthesis and modification of carbon-based electrodes for supercapacitor. J. Energy Storage 2022, 55, 105727. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, J.; Ji, D.; Li, J.; Zhao, S.; Zhao, Y.; Cai, Z.; He, X.; Sun, X. Carbonaceous electrode materials for supercapacitor: Preparation and surface functionalization. Front. Energy Res. 2023, 10, 957032. [Google Scholar] [CrossRef]
- Béguin, F.; Presser, V.; Balducci, A.; Frackowiak, E. Carbons and electrolytes for advanced supercapacitors. Adv. Mater. 2014, 26, 2219–2251. [Google Scholar] [CrossRef]
- Candelaria, S.L.; Shao, Y.; Zhou, W.; Li, X.; Xiao, J.; Zhang, J.-G.; Wang, Y.; Liu, J.; Li, J.; Cao, G. Nanostructured carbon for energy storage and conversion. Nano Energy 2012, 1, 195–220. [Google Scholar] [CrossRef]
- Balach, J.; Singh, H.K.; Gomoll, S.; Jaumann, T.; Klose, M.; Oswald, S.; Richter, M.; Eckert, J.; Giebeler, L. Synergistically Enhanced Polysulfide Chemisorption Using a Flexible Hybrid Separator with N and S Dual-Doped Mesoporous Carbon Coating for Advanced Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2016, 8, 14586–14595. [Google Scholar] [CrossRef]
- Xiao, J.; Li, H.; Zhang, H.; He, S.; Zhang, Q.; Liu, K.; Jiang, S.; Duan, G.; Zhang, K. Nanocellulose and its derived composite electrodes toward supercapacitors: Fabrication, properties, and challenges. J. Bioresour. Bioprod. 2022, 7, 245–269. [Google Scholar] [CrossRef]
- Cruz, O.F., Jr.; Gómez, I.C.; Escandell, M.M.; Rambo, C.R.; Silvestre-Albero, J. Activated carbon from polyurethane residues as molecular sieves for kinetic adsorption/separation of CO2/CH4. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129882. [Google Scholar] [CrossRef]
- Vargas, D.P.; Giraldo, L.; Moreno-Piraján, J.C. CO2 Adsorption on Activated Carbon Honeycomb-Monoliths: A Comparison of Langmuir and Tóth Models. Int. J. Mol. Sci. 2012, 13, 8388–8397. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, A.R.O.; Silvestre-Albero, J.; Maier, M.E.; Ricardo, N.M.P.S.; Cavalcante, C.L., Jr.; Luna, F.M.T. Sulfonated activated carbons as potential catalysts for biolubricant synthesis. Mol. Catal. 2020, 488, 110888. [Google Scholar] [CrossRef]
- Clohessy, J.; Kwapinski, W. Carbon-Based Catalysts for Biodiesel Production—A Review. Appl. Sci. 2020, 10, 918. [Google Scholar] [CrossRef] [Green Version]
- Zhai, Y.; Zhu, Z.; Dong, S. Carbon-Based Nanostructures for Advanced Catalysis. ChemCatChem 2015, 7, 2806–2815. [Google Scholar] [CrossRef]
- Karczmarska, A.; Adamek, M.; El Houbbadi, S.; Kowalczyk, P.; Laskowska, M. Carbon-Supported Noble-Metal Nanoparticles for Catalytic Applications—A Review. Crystals 2022, 12, 584. [Google Scholar] [CrossRef]
- Gómez-Cápiro, O.; Matschuk, K.; Schulzke, T.; Jiménez Concepción, R.; Arteaga-Pérez, L.E. Carbon Aerogel-Supported Iron for Gasification Gas Cleaning: Tars Decomposition. Catalysts 2022, 12, 391. [Google Scholar] [CrossRef]
- Zhu, C.; Li, H.; Fu, S.; Du, D.; Lin, Y. Highly efficient nonprecious metal catalysts towards oxygen reduction reaction based on three-dimensional porous carbon nanostructures. Chem. Soc. Rev. 2016, 45, 517–531. [Google Scholar] [CrossRef]
- Yang, Y.; Chiang, K.; Burke, N. Porous carbon-supported catalysts for energy and environmental applications: A short review. Catal. Today 2011, 178, 197–205. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Hogland, W.; Marques, M.; Sillanpää, M. An overview of the modification methods of activated carbon for its water treatment applications. Chem. Eng. J. 2013, 219, 499–511. [Google Scholar] [CrossRef]
- Bakly, S.; Al-Juboori, R.A.; Bowtell, L. Macadamia Nutshell Biochar for Nitrate Removal: Effect of Biochar Preparation and Process Parameters. C-J. Carbon Res. 2019, 5, 47. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Chen, Z.; Wang, S.; Chen, J.; Hu, B.; Shen, C.; Wang, X. Carbon-based nanocomposites for the elimination of inorganic and organic pollutants through sorption and catalysis strategies. Sep. Purif. Technol. 2023, 308, 122862. [Google Scholar] [CrossRef]
- Matisová, E.; Škrabáková, S. Carbon sorbents and their utilization for the preconcentration of organic pollutants in environmental samples. J. Chromatogr. A 1995, 707, 145–179. [Google Scholar] [CrossRef]
- Santoso, E.; Ediati, R.; Kusumawati, Y.; Bahruji, H.; Sulistiono, D.O.; Prasetyoko, D. Review on recent advances of carbon based adsorbent for methylene blue removal from waste water. Mater. Today Chem. 2020, 16, 100233. [Google Scholar] [CrossRef]
- Lotfy, H.R.; Roubík, H. Water purification using activated carbon prepared from agriculture waste—Overview of a recent development. Biomass Convers. Biorefin. 2021. [Google Scholar] [CrossRef]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef]
- Obey, G.; Adelaide, M.; Ramaraj, R. Biochar derived from non-customized matamba fruit shell as an adsorbent for wastewater treatment. J. Bioresour. Bioprod. 2022, 7, 109–115. [Google Scholar] [CrossRef]
- Beesley, L.; Moreno-Jiménez, E.; Gomez-Eyles, J.L.; Harris, E.; Robinson, B.; Sizmur, T. A review of biochars’ potential role in the remediation, revegetation and restoration of contaminated soils. Environ. Pollut. 2011, 159, 3269–3282. [Google Scholar] [CrossRef]
- Hadi, P.; To, M.-H.; Hui, C.-W.; Lin, C.S.K.; McKay, G. Aqueous mercury adsorption by activated carbons. Water Res. 2015, 73, 37–55. [Google Scholar] [CrossRef]
- Hassan, M.M.; Carr, C.M. Biomass-derived porous carbonaceous materials and their composites as adsorbents for cationic and anionic dyes: A review. Chemosphere 2021, 265, 129087. [Google Scholar] [CrossRef] [PubMed]
- Silvestre-Albero, A.; Ramos-Fernández, J.M.; Martínez-Escandell, M.; Sepúlveda-Escribano, A.; Silvestre-Albero, J.; Rodríguez-Reinoso, F. High saturation capacity of activated carbons prepared from mesophase pitch in the removal of volatile organic compounds. Carbon 2010, 48, 548–556. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, B.; Creamer, A.E.; Cao, C.; Li, Y. Adsorption of VOCs onto engineered carbon materials: A review. J. Hazard. Mater. 2017, 338, 102–123. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Arshad, N.; Hui, C.; Irshad, M.S.; Mushtaq, N.; Hussain, S.; Shah, M.; Naqvi, S.Z.H.; Rizwan, M.; Shahzad, N.; et al. Interfacial Photothermal Heat Accumulation for Simultaneous Salt Rejection and Freshwater Generation; an Efficient Solar Energy Harvester. Nanomaterials 2022, 12, 3206. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Bhaumik, A.; Wu, K.C.-W. Hierarchically porous carbon derived from polymers and biomass: Effect of interconnected pores on energy applications. Energy Environ. Sci. 2014, 7, 3574–3592. [Google Scholar] [CrossRef]
- Wu, S.-C.; Chang, P.-H.; Chou, S.-H.; Huang, C.-Y.; Liu, T.-C.; Peng, C.-H. Waffle-Like Carbons Combined with Enriched Mesopores and Highly Heteroatom-Doped Derived from Sandwiched MOF/LDH/MOF for High-Rate Supercapacitor. Nanomaterials 2020, 10, 2388. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, J.; Hyeon, T. Recent progress in the synthesis of porous carbon materials. Adv. Mater. 2006, 18, 2073–2094. [Google Scholar] [CrossRef]
- Marsh, H.; Rodriguez Reinoso, F. Activated Carbon, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Pekala, R.W. Organic aerogels from the polycondensation of resorcinol with formaldehyde. J. Mater. Sci. 1989, 24, 3221–3227. [Google Scholar] [CrossRef]
- Ryoo, R.; Joo, S.H.; Kruk, M.; Jaroniec, M. Ordered mesoporous carbons. Adv. Mater. 2001, 13, 677–681. [Google Scholar] [CrossRef]
- Mehdipour-Ataei, S.; Aram, E. Mesoporous Carbon-Based Materials: A Review of Synthesis, Modification, and Applications. Catalysts 2023, 13, 2. [Google Scholar] [CrossRef]
- Abebe, A.M.; Soraru, G.D.; Thothadri, G.; Andoshe, D.M.; Zambotti, A.; Ahmed, G.M.S.; Tirth, V.; Algahtani, A. Synthesis and Characterization of High Surface Area Transparent SiOC Aerogels from Hybrid Silicon Alkoxide: A Comparison between Ambient Pressure and Supercritical Drying. Materials 2022, 15, 1277. [Google Scholar] [CrossRef]
- Pierre Alain, C.; Pajonk Gérard, M. Chemistry of Aerogels and Their Applications. Chem. Rev. 2002, 102, 4243–4266. [Google Scholar] [CrossRef]
- Pons, A.; Casas, L.L.; Estop, E.; Molins, E.; Harris, K.D.M.; Xu, M. A new route to aerogels: Monolithic silica cryogels. J. Non-Cryst. Sol. 2012, 358, 461–469. [Google Scholar] [CrossRef]
- Mayer, S.T.; Kaschmitter, J.L.; Pekala, R.W. Method of Low Pressure and/or Evaporative Drying of Aerogel; USPTO: Alexandria, VA, USA, 1995; Volume 168, p. 5420. [Google Scholar]
- Bell, W.; Dietz, S. Mesoporous Carbons and Polymers. U.S. Patent 6,297,293 B1, 2 October 2001. [Google Scholar]
- Lee, K.T.; Oh, S.M. Novel synthesis of porous carbons with tunable pore size by surfactant-templated sol-gel process and carbonisation. Chem. Comm. 2002, 2, 2722–2723. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Fu, R.; Zhang, S.; Dresselhaus, M.S.; Dresselhaus, G. Preparation of low-density carbon aerogels by ambient pressure drying. Carbon 2004, 42, 2033–2039. [Google Scholar] [CrossRef]
- Bruno, M.M.; Cotella, N.G.; Miras, M.C.; Koch, T.; Seidler, S.; Barbero, C. Characterization of monolithic porous carbon prepared from resorcinol/formaldehyde gels with cationic surfactant. Colloids Surf. A Physicochem. Eng. Asp. 2010, 358, 13–20. [Google Scholar] [CrossRef]
- Tamborini, L.H.; Casco, M.E.; Militello, M.P.; Silvestre-Albero, J.; Barbero, C.A.; Acevedo, D.F. Successful application of a commercial cationic surfactant mixture (benzalkonium chloride) as porosity stabilizer in porous carbons fabrication. Colloids Surf. A Physicochem. Eng. Asp. 2016, 509, 449–456. [Google Scholar] [CrossRef] [Green Version]
- Balach, J.; Soldera, F.; Acevedo, D.F.; Mücklich, F.; Barbero, C.A. A direct and quantitative three-dimensional reconstruction of the internal structure of disordered mesoporous carbon with tailored pore size. Microsc. Microanal. 2013, 19, 745–750. [Google Scholar] [CrossRef]
- Jirglová, H.; Maldonado-Hódar, F.J. Chemical interactions of surface-active agents with growing resorcinol-formaldehyde gels. Langmuir 2010, 26, 16103–16109. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Bai, Y.; Wang, X.; Su, J. Supercapacitive behaviors of hierarchically porous carbons prepared by metal oxide/surfactant templates. J. Electrochem. Soc. 2012, 159, A431–A437. [Google Scholar] [CrossRef]
- Bruno, M.M.; Cotella, N.G.; Miras, M.C.; Barbero, C.A. A novel way to maintain resorcinol-formaldehyde porosity during drying: Stabilization of the sol-gel nanostructure using a cationic polyelectrolyte. Colloids Surf. A Physicochem. Eng. Asp. 2010, 362, 28–32. [Google Scholar] [CrossRef]
- Balach, J.; Tamborini, L.; Sapag, K.; Acevedo, D.F.; Barbero, C.A. Facile preparation of hierarchical porous carbons with tailored pore size obtained using a cationic polyelectrolyte as a soft template. Colloids Surf. A Physicochem. Eng. Asp. 2012, 415, 343–348. [Google Scholar] [CrossRef]
- Balach, J.; Miguel, F.; Soldera, F.; Acevedo, D.F.; Mücklich, F.; Barbero, C.A. A direct and quantitative image of the internal nanostructure of nonordered porous monolithic carbon using FIB nanotomography. J. Microsc. 2012, 246, 274–278. [Google Scholar] [CrossRef]
- Tamborini, L.; Militello, P.; Barbero, C.; Acevedo, D. Production of porous carbons from resorcinol-formaldehyde gels: Applications. In Handbook of Composites from Renewable Materials; Wiley: New York, NY, USA, 2017; Volume 1–8, pp. 175–196. [Google Scholar] [CrossRef]
- Balach, J.; Bruno, M.M.; Cotella, N.G.; Acevedo, D.F.; Barbero, C.A. Electrostatic self-assembly of hierarchical porous carbon microparticles. J. Power Sources 2012, 199, 386–394. [Google Scholar] [CrossRef]
- Tamborini, L.H.; Militello, M.P.; Balach, J.; Moyano, J.M.; Barbero, C.A.; Acevedo, D.F. Application of sulfonated nanoporous carbons as acid catalysts for Fischer esterification reactions. Arab. J. Chem. 2019, 12, 3172–3182. [Google Scholar] [CrossRef] [Green Version]
- Tamborini, L.H.; Casco, M.E.; Militello, M.P.; Silvestre-Albero, J.; Barbero, C.A.; Acevedo, D.F. Sulfonated porous carbon catalysts for biodiesel production: Clear effect of the carbon particle size on the catalyst synthesis and properties. Fuel Process. Technol. 2016, 149, 209–217. [Google Scholar] [CrossRef]
- Darmstadt, H.; Roy, C.; Kaliaguine, S.; Kim, T.-W.; Ryoo, R. Surface and pore structures of CMK-5 ordered mesoporous carbons by adsorption and surface spectroscopy. Chem. Mater. 2003, 15, 3300–3307. [Google Scholar] [CrossRef]
- Björk, E.M.; Militello, M.P.; Tamborini, L.H.; Coneo Rodriguez, R.; Planes, G.A.; Acevedo, D.F.; Moreno, M.S.; Odén, M.; Barbero, C.A. Mesoporous silica and carbon based catalysts for esterification and biodiesel fabrication—The effect of matrix surface composition and porosity. Appl. Catal. A-Gen. 2017, 533, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, C.; Proebstle, H.; Fricke, J. Carbon cloth-reinforced and activated aerogel films for supercapacitors. J. Non-Cryst. Solids 2001, 285, 277–282. [Google Scholar] [CrossRef]
- Caruso, R.A. Micrometer-to-Nanometer Replication of Hierarchical Structures by Using a Surface Sol–Gel Process. Angew.Chem. Int. Ed. 2004, 43, 2746–2748. [Google Scholar] [CrossRef]
- Gu, Y.; Huang, J. Natural Cellulosic Substance Derived Nano-structured Materials. In Nanostructured Biomaterials; Li, J., Ed.; Springer: Berlin, Germany, 2010. [Google Scholar] [CrossRef]
- Huang, C.-H.; Doong, R.-A. Sugarcane bagasse as the scaffold for mass production of hierarchically porous carbon monoliths by surface self-assembly. Microporous Mesoporous Mater. 2012, 147, 47–52. [Google Scholar] [CrossRef]
- Bruno, M.M.; Cotella, N.G.; Miras, M.C.; Barbero, C.A. Porous carbon–carbon composite replicated from a natural fibre. Chem. Commun. 2005, 47, 5896. [Google Scholar] [CrossRef]
- Pandey, J.K.; Ahn, S.H.; Lee, C.S.; Mohanty, A.K.; Misra, M. Recent advances in the application of natural fiber based composites. Macromol. Mater. Eng. 2010, 295, 975–989. [Google Scholar] [CrossRef]
- Biagiotti, J.; Puglia, D.; Kenny, J.M. A review on natural fibre-based composites—Part I: Structure, processing and properties of vegetable fibres. J. Nat. Fibers 2004, 1, 37–68. [Google Scholar] [CrossRef]
- Adekunle, A.A.; Adekunle, I.M.; Opafola, O.T.; Ogundare, I.; Adeyeye, A. Evaluation of strength characteristics of fibre reinforced concrete: A case study of glass and sisal fibres. Herit. Sustain. Dev. 2022, 4, 27–31. [Google Scholar] [CrossRef]
- Comroe, M.L.; Kolasinski, K.W.; Saha, D. Direct Ink 3D Printing of Porous Carbon Monoliths for Gas Separations. Molecules 2022, 27, 5653. [Google Scholar] [CrossRef]
- Klepel, O.; Danneberg, N. Porous Carbon Monoliths Made from Cellulose and Starch. C-J. Carbon Res. 2020, 6, 32. [Google Scholar] [CrossRef]
- Rodriguez, R.C.; Moncada, A.B.; Acevedo, D.F.; Planes, G.A.; Miras, M.C.; Barbero, C.A. Electroanalysis using modified hierarchical nanoporous carbon materials. Faraday Discuss. 2014, 164, 147–173. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.M.; Corti, H.R.; Balach, J.; Cotella, N.G.; Barbero, C.A. Hierarchical porous materials: Capillaries in nanoporous carbon. Funct. Mater. Lett. 2009, 2, 135–138. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, C.; Wang, H.; Jian, M.; Hao, X.; Zhang, Y. Carbonized Cotton Fabric for High-Performance Wearable Strain Sensors. Adv. Funct. Mater. 2017, 27, 1604795. [Google Scholar] [CrossRef]
- Chang, S.; Li, J.; He, Y.; Liu, H.; Cheng, B. A high-sensitivity and low-hysteresis flexible pressure sensor based on carbonized cotton fabric. Sens. Actuator A Phys. 2019, 294, 45–53. [Google Scholar] [CrossRef]
- Ko, Y.; Vu, C.C.; Kim, J. Carbonized Cotton Fabric-Based Flexible Capacitive Pressure Sensor Using a Porous Dielectric Layer with Tilted Air Gaps. Sensors 2021, 21, 3895. [Google Scholar] [CrossRef]
- Wu, R.; Ma, L.; Patil, A.; Meng, Z.; Liu, S.; Hou, C.; Zhang, Y.; Yu, W.; Guo, W.; Liu, X.Y. Graphene decorated carbonized cellulose fabric for physiological signal monitoring and energy harvesting. J. Mater. Chem. A 2020, 8, 12665–12673. [Google Scholar] [CrossRef]
- Macias-Garcia, A.; Cuerda-Correa, E.; Olivares-Marinb, M.; Diaz-Paralejo, A.Y.; Diaz-Dieza, M.A. Development and characterization of carbon-honeycomb monoliths from kenaf natural fibers: A preliminary study. Ind. Crops Prod. 2012, 35, 105–110. [Google Scholar] [CrossRef]
- Ma, G.; Guo, D.; Sun, K.; Peng, H.; Yang, Q.; Zhou, X.; Zhao, X.; Lei, Z. Cotton-based porous activated carbon with a large specific surface area as an electrode material for high-performance supercapacitors. RSC Adv. 2015, 5, 64704–64710. [Google Scholar] [CrossRef]
- Du, J.; Liu, L.; Hu, Z.; Yu, Y.; Zhang, Y.; Hou, S.; Chen, A. Raw-Cotton-Derived N-Doped Carbon Fiber Aerogel as an Efficient Electrode for Electrochemical Capacitors. ACS Sustain. Chem. Eng. 2018, 6, 4008–4015. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. Measurement of hardness and elastic modulus by instrumented indentation: Advances in understanding and refinements to methodology. J. Mater. Res. 2004, 19, 1. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 2000; pp. 17–18. [Google Scholar]
- Garay, F.; Barbero, C.A. Charge neutralization process of mobile species at any distance from the electrode/solution interface. 1. Theory and simulation of concentration and concentration gradients developed during potentiostatic conditions. Anal. Chem. 2006, 78, 6733–6739. [Google Scholar] [CrossRef]
- Islam, M.; Weidler, P.G.; Mager, D.; Korvink, J.G.; Martinez-Duarte, R. Comparing Carbon Origami from Polyaramid and Cellulose Sheets. Micromachines 2022, 13, 503. [Google Scholar] [CrossRef]
- Mironova, M.; Makarov, I.; Golova, L.; Vinogradov, M.; Shandryuk, G.; Levin, I. Improvement in Carbonization Efficiency of Cellulosic Fibres Using Silylated Acetylene and Alkoxysilanes. Fibers 2019, 7, 84. [Google Scholar] [CrossRef] [Green Version]
- Leclère, M.; Lejeune, M.; Dupont, L.; Barrès, A.-L.; Renault, S.; Dolhem, F.; Poizot, P. Pyrolysis reaction of squaric acid: A one-step method for producing expanded foam of mesoporous carbon. Mater. Lett. 2014, 137, 233–236. [Google Scholar] [CrossRef]
- Li, T.; Cao, M.; Liang, J.; Xie, X.; Du, G. Mechanism of Base-Catalyzed Resorcinol-Formaldehyde and Phenol-Resorcinol-Formaldehyde Condensation Reactions: A Theoretical Study. Polymers 2017, 9, 426. [Google Scholar] [CrossRef] [PubMed]
- Fields, J.S.; Swain, M.V. The indentation characterization of the mechanical properties of various carbon materials: Glassy carbon, coke and pyrolytic graphite. Carbon 1996, 34, 1357–1366. [Google Scholar] [CrossRef]
- Fleck, N.A.; Otoyo, H.; Needleman, A. Indentation on porous solids. Int. J. Solids Struct. 1992, 13, 1613–1636. [Google Scholar] [CrossRef]
- Huang, J.; Kunitake, T. Nano-precision replication of natural cellulosic substances by metal oxides. J. Am. Chem. Soc. 2003, 125, 11834–11835. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Wannassi, B.; Kanan, M.; Hariz, I.B.; Assaf, R.; Abusaq, Z.; Ben Hassen, M.; Aljazzar, S.; Zahran, S.; Khouj, M.T.; Barham, A.S. Cotton Spinning Waste as a Microporous Activated Carbon: Application to Remove Sulfur Compounds in a Tunisian Refinery Company. Sustainability 2023, 15, 654. [Google Scholar] [CrossRef]
- Evans, R.J.; Wang, D.; Agblevor, F.A.; Chum, H.L.; Baldwin, S.D. Mass spectrometric studies of the thermal decomposition of carbohydrates using 13C-labeled cellulose and glucose. Carbohydr. Res. 1996, 281, 219–235. [Google Scholar] [CrossRef]
- Park, B.-I.; Bozzelli, J.W.; Booty, M.R.; Bernhard, M.J.; Mesuere, K.; Pettigrew, C.A.; Shi, J.-C.; Simonich, S.L. Polymer pyrolysis and oxidation studies in a continuous feed and flow reactor: Cellulose and polystyrene. Environ. Sci. Technol. 1999, 33, 2584–2592. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, L.; Yang, Y.; Li, Y.; Ren, S.; Dong, L.; Lei, T. Fast Pyrolysis of Cellulose and the Effect of a Catalyst on Product Distribution. Int. J. Environ. Res. Public Health 2022, 19, 16837. [Google Scholar] [CrossRef]
- Chang, C.; Tackett, J.R. Characterization of phenolic resins with thermogravimetry-mass spectrometry. Thermochim. Acta 1991, 192, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Barbero, C.; Silber, J.J.; Sereno, L. Studies of surface-modified glassy carbon electrodes obtained by electrochemical treatment. Its effect on Ru(bpy)2+3 adsorption and the electron transfer rates of the Fe2+/Fe3+ couple, J. Electroanal. Chem. 1988, 248, 321–340. [Google Scholar] [CrossRef]
- He, X.; Chen, Q.; Mao, X.; Liu, W.; Zhou, Y.; Yang, W.; Yang, Y.; Xu, J. Pseudocapacitance electrode and asymmetric supercapacitor based on biomass juglone/activated carbon composites. RSC Adv. 2019, 9, 30809–30814. [Google Scholar] [CrossRef] [Green Version]
- Barbero, C.; Kotz, R. Electrochemical activation of glassy carbon. Spectroscopic ellipsometry of surface phase formation. J. Electrochem. Soc. 1993, 140, 1–6. [Google Scholar] [CrossRef]
- Planes, G.A.; Miras, M.C.; Barbero, C.A. Double layer properties of carbon aerogel electrodes measured by probe beam deflection and AC impedance techniques. Chem. Comm. 2005, 16, 2146–2148. [Google Scholar] [CrossRef]
- Kötz, R.; Carlen, M. Principles and applications of electrochemical capacitors. Electrochim. Acta 2000, 45, 2483–2498. [Google Scholar] [CrossRef]
- dos Reis, G.S.; Larsson, S.H.; de Oliveira, H.P.; Thyrel, M.; Claudio Lima, E. Sustainable Biomass Activated Carbons as Electrodes for Battery and Supercapacitors—A Mini-Review. Nanomaterials 2020, 10, 1398. [Google Scholar] [CrossRef] [PubMed]
- Jurewicz, K.; Frackowiak, E.; Béguin, F. Towards the mechanism of electrochemical hydrogen storage in nanostructured carbon materials. Appl. Phys. A 2004, 78, 981–987. [Google Scholar] [CrossRef]
- Eliad, L.; Salitra, G.; Soffer, A.; Aurbach, S. On the mechanism of selective electroadsorption of protons in the pores of carbon molecular sieves. Langmuir 2005, 21, 3198–3202. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S. Glassy Carbon: A Promising Material for Micro- and Nanomanufacturing. Materials 2018, 11, 1857. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.; Zhang, J.; Deng, H.; Du, Y.; Shi, X. Chitin derived nitrogen-doped porous carbons with ultrahigh specific surface area and tailored hierarchical porosity for high performance supercapacitors. J. Bioresour. Bioprod. 2021, 6, 142–151. [Google Scholar] [CrossRef]
- Barbero, C.A. Ion exchange at the electrode/electrolyte interface studied by probe beam deflection techniques. PCCP 2005, 7, 1885–1899. [Google Scholar] [CrossRef]
- Hu, M.; Cui, C.; Shi, C.; Wu, Z.-S.; Yang, J.; Cheng, R.; Guang, T.; Wang, H.; Lu, H.; Wang, X. High-Energy-Density Hydrogen-Ion-Rocking-Chair Hybrid Supercapacitors Based on Ti3C2 Tx MXene and Carbon Nanotubes Mediated by Redox Active Molecule. ACS Nano 2019, 13, 6899–6905. [Google Scholar] [CrossRef] [PubMed]
- Koetz, R.; Barbero, C.; Haas, O. Probe Beam Deflection for the Analysis of Ion Fluxes at the Solid/Liquid Interface. Ber. Bunsen-Ges. Phys. Chem. 1993, 97, 427–430. [Google Scholar] [CrossRef]
- Garay, F.; Barbero, C.A. Charge neutralization process of mobile species at any distance from the electrode/solution interface. 2. Concentration gradients during potential pulse experiments. Anal. Chem. 2006, 78, 6740–6746. [Google Scholar] [CrossRef]
- Barbero, C.; Miras, M.C.; Kötz, R. Electrochemical mass transport studied by probe beam deflection: Potential step experiments. Electrochim. Acta 1992, 37, 429–437. [Google Scholar] [CrossRef]
- Zhao, X.; Aoki, K.J.; Chen, J.; Nishiumi, T. Examination of the Gouy-Chapman theory for double layer capacitance in deionized latex suspensions. RSC Adv. 2014, 4, 63171–63181. [Google Scholar] [CrossRef]
- Martínez-Cifuentes, M.; Salazar, R.; Ramírez-Rodríguez, O.; Weiss-López, B.; Araya-Maturana, R. Experimental and Theoretical Reduction Potentials of Some Biologically Active ortho-Carbonyl para-Quinones. Molecules 2017, 22, 577. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; He, M.; Han, J.; Sun, Y.; Jiang, H.; Li, Z.; Li, Y.; Zhang, H. Recent Advances in Capacitive Deionization: Research Progress and Application Prospects. Sustainability 2022, 14, 14429. [Google Scholar] [CrossRef]
- Torop, J.; Arulepp, M.; Leis, J.; Punning, A.; Johanson, U.; Palmre, V.; Aabloo, A. Nanoporous Carbide-Derived Carbon Material-Based Linear Actuators. Materials 2010, 3, 9–25. [Google Scholar] [CrossRef] [Green Version]















| Material | Elastic Modulus (GPa) | Hardness (GPa) |
|---|---|---|
| Hierarchical carbon: c(RF@F) | 14.2 ± 2.03 | 2.21 ± 0.34 |
| Mesoporous carbon: c(RFCTAB) | 6.6 ± 0.34 | 0.93 ± 0.07 |
| Carbon Material | Surface Area (m2 g−1) | Specific Capacitance (CV, F g−1) * | Specific Capacitance (EIS, F g−1) # | Reference |
|---|---|---|---|---|
| c(RF stab. CTAB) | 536 | 154 | 194 | [56] |
| c(RF stab. CTAB) | 671 | 145 | 145 | [47] |
| c(RF stab. PDAMAC) | 725 | 210 | -- | [52] |
| c(RF stab PDAMAC) | 675 | 97 | 142 | [53] |
| c(RF stab. CTAC) | 453 | 135 | -- | [44] |
| c(RF stab. BzACl) | 535 | -- | 179 | [48] |
| c(RF stab CTAB + NiO) | 637 | 271 | 216 | [51] |
| c(chitin) activated (NaOH) | 2631 | 227 & | -- | [105] |
| c(RF stab. Cel. Fibers) | 558 | 182 | 160 | This work |
| c(RF stab CTAB + Cel. Fibers) | -- | 130 | -- | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruno, M.M.; Cotella, N.G.; Barbero, C.A. Hierarchical Biobased Macroporous/Mesoporous Carbon: Fabrication, Characterization and Electrochemical/Ion Exchange Properties. Materials 2023, 16, 2101. https://doi.org/10.3390/ma16052101
Bruno MM, Cotella NG, Barbero CA. Hierarchical Biobased Macroporous/Mesoporous Carbon: Fabrication, Characterization and Electrochemical/Ion Exchange Properties. Materials. 2023; 16(5):2101. https://doi.org/10.3390/ma16052101
Chicago/Turabian StyleBruno, Mariano M., N. Gustavo Cotella, and Cesar A. Barbero. 2023. "Hierarchical Biobased Macroporous/Mesoporous Carbon: Fabrication, Characterization and Electrochemical/Ion Exchange Properties" Materials 16, no. 5: 2101. https://doi.org/10.3390/ma16052101