Crystallization of Cristobalite in Sodium Borosilicate Glass in the Presence of Cr2O3
Abstract
:1. Introduction
2. Materials and Methods
2.1. Glass Synthesis
2.2. X-ray Powder Diffractometry (XRD)
2.3. Scanning Electron Microscopy (SEM)
2.4. Electron Paramagnetic Resonance (EPR)
2.5. Fourier-Transform Infrared (FTIR) Spectroscopy
2.6. X-ray Photoelectron Spectroscopy (XPS)
2.7. Solid-State Nuclear Magnetic Resonance (NMR)
3. Results
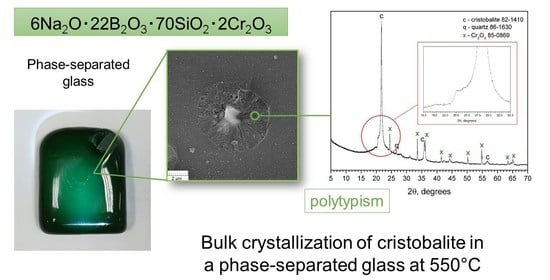
3.1. XRD Results
3.2. SEM Results
3.3. EPR Results
3.4. FTIR Spectroscopy Results
| Band Position | Glasses’ Designations | Band Assignment | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Quenched | Annealed | 550 °C, 24 h | 550 °C, 96 h | 6/70 | Cristobalite | Tridymite | |||
| 550–400 | 461 | 464 | 463 | 464 | 459 | 480 | 476 | δSiO4 bending vibrations in the silicon-oxygen groups | [53,54,55] |
| 650–550 | 565 | ||||||||
| 582 | 582 | 582 | 582 | Cr2O3 typical of O-Cr-O vibrations | [56,57] | ||||
| 633 | 632 | 626 | 625 | 621 | 648 | Si−O−Si, symmetrical stretching vibrations of the individual SiO4 tetrahedra of the polymerized structure of framework silicates such as α-cristobalite | [58] | ||
| 800–650 | 673 | 675 | 675 | 676 | 675 | 679 | B-O-Si stretching; δBO3 bending vibrations of bridging oxygen between trigonal BO3 groups | [15,53,59,60] | |
| 700 | 699 | 699 | 699 | 700 | 696 | δBO3; bending vibrations of B-O-B linkage in borate network | [53,61,62,63,64] | ||
| 804 | 800 | 800 | 793 | 799 | 793 | 789 | Bending motions of the Si-O-Si bridges; α-cristobalite | [53,58,65] | |
| 1200–800 | 922 | 922 | 924 | 910 | 924 | B-O-Si stretching mode ν (B–O–Si) | [66] | ||
| 1090 | 1092 | 1093 | 1095 | 1092 | 1096 | 1103 | antisymmetric stretching vibrations of the O–Si–O bonds in SiO4 tetrahedral units for tetrahedra with three bridging oxygen ions (Q3) | [53,55] | |
| 1198 | |||||||||
| 1500–1200 | 1396 | 1397 | 1399 | 1399 | 1396 | B-O stretching vibrations involving B-O-B linkages associated with triangularly and tetrahedrally coordinated boron atoms; bending and stretching modes of the B–O bonds in the BO3 triangles | [62,64] | ||
| 2000–1500 | 1630 | 1630 | 1623 | 1636 | 1636 | OH symmetrical bending deformation | [67] | ||
3.5. XPS Results
3.6. NMR Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdelghany, A.M.; Elbatal, F.H.; Elbatal, H.A.; EzzElDin, F.M. Optical and FTIR structural studies of CoO-doped sodium borate, sodium silicate and sodium phosphate glasses and effects of gamma irradiation—A comparative study. J. Mol. Struct. 2014, 1074, 503–510. [Google Scholar] [CrossRef]
- Moustafa, F.A.; Fayad, A.M.; Ezz-Eldin, F.M.; El-Kashif, I. Effect of gamma radiation on ultraviolet, visible and infrared studies of NiO, Cr2O3 and Fe2O3-doped alkali borate glasses. J. Non. Cryst. Solids 2013, 376, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, F. Study the effect of alkali/alkaline earth addition on the environment of borochromate glasses by means of spectroscopic analysis. J. Alloys Compd. 2014, 586, 605–610. [Google Scholar] [CrossRef]
- Katase, T.; Ohta, H. Surface charge accumulation and electrochemical protonation of transition metal oxides using water-infiltrated nanoporous glass. Semicond. Sci. Technol. 2019, 34, 123001. [Google Scholar] [CrossRef]
- Salman, S.M.; Salama, S.N.; Mahdy, E.A. Contribution of some transition metal oxides to crystallization and electro-thermal properties of glass-ceramics. Ceram. Int. 2020, 46, 13724–13731. [Google Scholar] [CrossRef]
- Ahmed, M.R.; Ashok, B.; Ahmmad, S.K.; Hameed, A.; Chary, M.N.; Shareefuddin, M. Infrared and Raman spectroscopic studies of Mn2+ ions doped in strontium alumino borate glasses: Describes the role of Al2O3. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 210, 308–314. [Google Scholar] [CrossRef]
- Bhogi, A.; Kistaiah, P. Spectroscopic properties of alkali alkaline earth borate glasses doped with Fe3+ ions. J. Aust. Ceram. Soc. 2020, 56, 127–138. [Google Scholar] [CrossRef]
- Banagar, A.V.; Prashant Kumar, M.; Nagaraja, N. Effect of Mixed Transition Metal Ions in B2O3-V2O5-MoO3 Glass System. J. Electron. Mater. 2020, 49, 7370–7378. [Google Scholar] [CrossRef]
- Stepanov, S.A.; Nikonorov, N.V.; Aseev, V.A.; Zapalova, S.S. Spectral-luminescent properties of trivalent chromium ions in glass of the system K2O-Al2O3-B2O3. Glass Phys. Chem. 2015, 41, 151–156. [Google Scholar]
- Aktas, B.; Yalcin, S.; Dogru, K.; Uzunoglu, Z.; Yilmaz, D. Structural and radiation shielding properties of chromium oxide doped borosilicate glass. Radiat. Phys. Chem. 2019, 156, 144–149. [Google Scholar] [CrossRef]
- Wang, Z.; Ye, R.; Huang, F.; Fei, E.; Hua, Y.; Zhang, J.; Xu, S. Modulating energy transfer between transition metal and rare earth ions in nanostructured glass to promote ultrabroadband infrared emission. J. Alloys Compd. 2019, 784, 513–518. [Google Scholar] [CrossRef]
- Van Die, A.; Leenaers, A.C.H.I.; Blasse, G.; van der Weg, W.F. Germanate glasses as hosts for luminescence of Mn2+ and Cr3+. J. Non. Cryst. Solids 1988, 99, 32–44. [Google Scholar] [CrossRef]
- Babkina, A.N.; Gorbachev, A.D.; Zyryanova, K.S.; Nikonorov, N.V.; Nuryev, R.K.; Stepanov, S.A. A study of the effect of lithium oxide on the spectral properties of potassium-aluminoborate glass activated by chromium ions. Opt. Spectrosc. (English Transl. Opt. i Spektrosk. 2017, 123, 369–374. [Google Scholar] [CrossRef]
- Babkina, A.N.; Zyryanova, K.S.; Agafonova, D.A.; Nuryev, R.K.; Kolobkova, E.V.; Ignatiev, A.I. Crystallization kinetics and luminescent properties of chromium-doped borate glass-ceramics. J. Phys. Conf. Ser. 2019, 1400, 066002. [Google Scholar] [CrossRef]
- Ebrahimi, E.; Rezvani, M. Optical and structural investigation on sodium borosilicate glasses doped with Cr2O3. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 190, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Kashif, I. Magnetic susceptibility of lithium borosilicate glasses containing metal oxide. J. Mater. Sci. Mater. Electron. 1990, 1, 49–50. [Google Scholar] [CrossRef]
- Ravi Kumar, G.; Gopi Krishna, M.; Rao, M.C. Cr3+ doped NaF–ZrO2–B2O3–SiO2 glass ceramic materials for optoelectronic device application. Optik 2018, 173, 78–87. [Google Scholar] [CrossRef]
- Samdani; Ramadevudu, G.; Chary, M.N.; Shareefuddin, M. Physical and spectroscopic studies of Cr3+ doped mixed alkaline earth oxide borate glasses. Mater. Chem. Phys. 2017, 186, 382–389. [Google Scholar] [CrossRef]
- Aseev, V.A.; Zhukov, S.N.; Kuleshov, N.V.; Kuril’chik, S.V.; Mudryi, A.V.; Nikonorov, N.V.; Rokhmin, A.S.; Yasyukevich, A.S. Spectral luminescence characteristics of forsterite nano glass ceramics doped with chromium ions. Opt. Spectrosc. 2015, 118, 146–150. [Google Scholar] [CrossRef]
- Abramov, A.N.; Yashkov, M.V.; Guryanov, A.N.; Melkumov, M.A.; Dvoretskii, D.A.; Bufetov, I.A.; Iskhakova, L.D.; Koltashev, V.V.; Kachenyuk, M.N.; Torsunov, M.F. Fabrication and optical characterization of silica fibers with a chromium- and alumina-doped core. Inorg. Mater. 2014, 50, 1283–1288. [Google Scholar] [CrossRef]
- Santhan Kumar, J.; Lakshmi Kumari, J.; Subba Rao, M.; Cole, S. EPR, optical and physical properties of chromium ions in CdO-SrO-B 2O3-SiO2 (CdSBSi) glasses. Opt. Mater. 2013, 35, 1320–1326. [Google Scholar] [CrossRef]
- Ulyashenko, A.M.; Nikonorov, N.V.; Przhevuskii, A.K. Forsterite nano glass-ceramics doped with Cr4+ ions for fiber lasers and amplifiers. Bull. Russ. Acad. Sci. Phys. 2007, 71, 159–163. [Google Scholar] [CrossRef]
- Agafonova, D.A.; Babkina, A.N.; Zyryanova, K.S.; Ignatiev, A.I.; Nikonorov, N.V.; Oreshkina, K.V. Investigation of Spectral Properties of Potassium Aluminoborate Glasses Doped with Chromium. Phys. Solid State 2019, 61, 826–829. [Google Scholar] [CrossRef]
- Reisfeld, R. Potential uses of chromium(III)-doped transparent glass ceramics in tunable lasers and luminescent solar concentrators. Mater. Sci. Eng. 1985, 71, 375–382. [Google Scholar] [CrossRef]
- Van Die, A.; Blasse, G.; Van Der Weg, W.F. Luminescence properties of Cr3+ doped borate glasses: Influence of crystal field strength and microcrystallite formation. Mater. Chem. Phys. 1986, 14, 513–523. [Google Scholar] [CrossRef]
- Yusub, S.; Krishna Rao, D. The role of chromium ions on dielectric and spectroscopic properties of Li2O-PbO-B2O3-P2O5 glasses. J. Non. Cryst. Solids 2014, 398–399, 1–9. [Google Scholar] [CrossRef]
- Aygün, B.; Şakar, E.; Cinan, E.; Yorgun, N.Y.; Sayyed, M.I.; Agar, O.; Karabulut, A. Development and production of metal oxide doped glasses for gamma ray and fast neutron shielding. Radiat. Phys. Chem. 2020, 174, 108897. [Google Scholar] [CrossRef]
- Sandu, V.; Nicolescu, M.S.; Kuncser, V.; Damian, R.; Sandu, E. Magnetic glass-ceramics. J. Adv. Ceram. 2012, 1, 138–143. [Google Scholar] [CrossRef] [Green Version]
- Mòulkoç, B.; Knowles, K.M.; Jansen, H.V.; Ter Brake, H.J.M.; Elwenspoek, M.C. Surface devitrification and the growth of cristobalite in borofloat ® (Borosilicate 8330) glass. J. Am. Ceram. Soc. 2010, 93, 2713–2719. [Google Scholar] [CrossRef]
- Butler, M.A.; Dyson, D.J. The Quantification of Different Forms of Cristobalite in Devitrified Alumino-Silicate Ceramic Fibres. J. Appl. Crystallogr. 1997, 30, 467–475. [Google Scholar] [CrossRef]
- Polyakova, I.G. The main silica phases and some of their properties. In Glass: Selected Properties and Crystallization; Walter de Gruyter: Berlin, Germany, 2014; pp. 197–268. [Google Scholar] [CrossRef]
- Şan, O.; Özgür, C. Investigation of a high stable β-cristobalite ceramic powder from CaO-Al2O3-SiO2 system. J. Eur. Ceram. Soc. 2009, 29, 2945–2949. [Google Scholar] [CrossRef]
- Pascova, R.; Avdeev, G.; Gutzow, I.; Penkov, I.; Ludwig, F.P.; Schmelzer, J.W.P. Refractory Alkali-Free Cristobalite Glass-Ceramics: Activated Reaction Sinter-Crystallization Synthesis and Properties. Int. J. Appl. Glass Sci. 2012, 3, 75–87. [Google Scholar] [CrossRef]
- Bruhns, P.; Fischer, R.X. Crystallization of cristobalite and tridymite in the presence of vanadium. Eur. J. Mineral. 2000, 12, 615–624. [Google Scholar] [CrossRef]
- Musgraves, J.D.; Hu, J.; Calvez, L. Springer Handbook of Glass; Springer: Berlin/Heidelberg, Germany, 2019; p. 1851. [Google Scholar] [CrossRef]
- Varshneya, A.K. Fundamentals of Inorganic Glasses; Elsevier: Amsterdam, The Netherlands, 1994. [Google Scholar]
- Wen, H.; Tanner, P.A. Optical properties of 3d transition metal ion-doped sodium borosilicate glass. J. Alloys Compd. 2015, 625, 328–335. [Google Scholar] [CrossRef]
- Konon, M.Y.; Stolyar, S.V.; Dikaya, L.F.; Polyakova, I.G.; Drozdova, I.A.; Antropova, T.V. Physicochemical properties of glasses of the Na2O-B2O3-SiO2-Fe2O3 system in the 8Na2O/70SiO2 section. Glass Phys. Chem. 2015, 41, 116–121. [Google Scholar] [CrossRef]
- Konon, M.Y.; Stolyar, S.V.; Drozdova, I.A.; Polyakova, I.G.; Dikaya, L.F. Phase-separated structure and properties of glasses in the (16–x)Na2O–14B2O3–70SiO2–xFe2O3 system. Glass Phys. Chem. 2017, 43, 389–394. [Google Scholar] [CrossRef]
- Konon, M.Y.; Stolyar, S.V.; Anfimova, I.N.; Polyakova, I.G.; Dikaya, L.F. Physicochemical Properties of Glasses of the Na2O–B2O3–SiO2–Fe2O3 System in the 6Na2O/70SiO2 Section. Glass Phys. Chem. 2018, 44, 495–497. [Google Scholar] [CrossRef]
- Konon, M.Y.; Polyakova, I.G.; Stolyar, S.V.; Anfimova, I.N. Crystallization in Glasses of the Na2O–B2O3–SiO2–Fe2O3 System with a Different SiO2 Content. Glass Phys. Chem. 2020, 46, 646–649. [Google Scholar] [CrossRef]
- Ehrt, D.; Reiss, H.; Vogel, W. Einbau und Verteilung von Fe2O3 auf die Mikrophasen in Gründglasern des Systems Na2O–B2O3–SiO2. Silikattechnik 1976, 27, 304–309. [Google Scholar]
- Jean, J.-H.; Gupta, T.K. Alumina as a Devitrification Inhibitor during Sintering of Borosilicate Glass Powders. J. Am. Ceram. Soc. 1993, 76, 2010–2016. [Google Scholar] [CrossRef]
- Kagawa, Y.; Kogo, Y.; Hatta, H. Thermal Expansion Behavior of the Si3N4-Whisker-Reinforced Soda-Borosilicate glass matrix composite. J. Am. Ceram. Soc. 1989, 72, 1092–1094. [Google Scholar] [CrossRef]
- Konon, M.Y.; Polyakova, I.G.; Saratovskii, A.S.; Danilovich, D.P.; Anfimova, I.N. Crystallization of Sodium Borosilicate Glass with the Addition of Cr2O3. Glass Phys. Chem. 2023, 49, 199–202. [Google Scholar] [CrossRef]
- Padlyak, B.V.; Ryba-Romanowski, W.; Lisiecki, R.; Adamiv, V.T.; Burak, Y.V.; Teslyuk, I.M. Synthesis, EPR and optical spectroscopy of the Cr-doped tetraborate glasses. Opt. Mater. 2012, 34, 2112–2119. [Google Scholar] [CrossRef]
- Lin, C.; Liu, J.; Han, L.; Gui, H.; Song, J.; Li, C.; Liu, T.; Lu, A. Study on the structure, thermal and optical properties in Cr2O3-incorporated MgO-Al2O3-SiO2-B2O3 glass. J. Non. Cryst. Solids 2018, 500, 235–242. [Google Scholar] [CrossRef]
- Durville, F.; Champagnon, B.; Duval, E.; Boulon, G. Laser spectroscopy of spinel microcrystallites in a Cr3+ doped silicate glass. J. Phys. Chem. Solids 1985, 46, 701–707. [Google Scholar] [CrossRef]
- Ardelean, I.; Ilonca, G.; Peteanu, M.; Barbos, E. EPR and magnetic susceptibility studies of xCr20 − (I-x)3B = O -PbO glasses. J. Mater. Sci. 1996, 17, 1988–1996. [Google Scholar] [CrossRef]
- Barbu, A.; Bratu, I.; Ardelean, I.; Cozar, O. Photoacoustic studies of xCr2O3(1-x)[2P2O5*Na2O] glasses. J. Mol. Struct. 1995, 349, 191–194. [Google Scholar] [CrossRef]
- Mazurin, O.V.; Roskova, G.P.; Antropova, T.V. On the Effect of Temperature on the Directions of Tie-lines in the Immiscibility Region of the Sodium Borosilicate System (O vliyanii temperatury na napravleniya konod v oblasti likvacii natrievoborosilikatnoi sistemy—In Russian). Glass Phys. Chem. 1981, 7, 560–569. [Google Scholar]
- Kumar, V.; Pandey, O.P.; Singh, K. Effect of A2O3 (A = La, Y, Cr, Al) on thermal and crystallization kinetics of borosilicate glass sealants for solid oxide fuel cells. Ceram. Int. 2010, 36, 1621–1628. [Google Scholar] [CrossRef]
- Glazkova, Y.S.; Kalmykov, S.N.; Presnyakov, I.A.; Stefanovskaya, O.I.; Stefanovsky, S.V. The structural state of iron in multicomponent aluminum iron borosilicate glass depending on their composition and synthesis conditions. Glass Phys. Chem. 2015, 41, 367–377. [Google Scholar] [CrossRef]
- Protasova, L.G.; Kosenko, V.G. Effect of additives on the structure and physicochemical properties of sodium-borosilicate glasses. Glass Ceram. 2003, 60, 164–165. [Google Scholar] [CrossRef]
- Akatov, A.A.; Nikonov, B.S.; Omel’Yanenko, B.I.; Stefanovsky, S.V.; Marra, J.C. Structure of borosilicate glassy materials with high concentrations of sodium, iron, and aluminum oxides. Glass Phys. Chem. 2009, 35, 245–259. [Google Scholar] [CrossRef]
- Sackey, J.; Morad, R.; Bashir AK, H.; Kotsedi, L.; Kaonga, C.; Maaza, M. Bio-synthesised black α-Cr2O3 nanoparticles; experimental analysis and density function theory calculations. J. Alloys Compd. 2021, 850, 156671. [Google Scholar] [CrossRef]
- Chromium Oxide. Available online: https://webbook.nist.gov/cgi/cbook.cgi?ID=C1308389&Units=SI&Mask=80#IR-Spec (accessed on 31 May 2023).
- Qiao, Z.; Liu, Q.; Zhang, S.; Wu, Y. The mineralogical characteristics between opaline silica in bentonite and α-cristobalite. Solid State Sci. 2019, 96, 105948. [Google Scholar] [CrossRef]
- Music, S.; Furic, K.; Bajs, Z.; Mohacek, V. Spectroscopic characterization of alkali borosilicate glasses containing iron ions. J. Mater. Sci. 1992, 27, 5269–5275. [Google Scholar] [CrossRef]
- Sanad, A.M.; Moustafa, F.A.; El-Sharkawy, A.A.; Mostafa, A.G.; El-Saghier, A.A.; Kashif, I. Infrared spectra, X-ray investigations and electrical conductivity of lithium borosilicate glasses containing transition metal oxides. J. Mater. Sci. 1988, 23, 1553–1557. [Google Scholar] [CrossRef]
- Konon, M.; Antropova, T.; Zolotov, N.; Simonenko, T.; Simonenko, N.; Brazovskaya, E.; Kreisberg, V.; Polyakova, I. Chemical durability of the iron-containing sodium borosilicate glasses. J. Non. Cryst. Solids 2022, 584, 121519. [Google Scholar] [CrossRef]
- Eremyashev, V.E.; Mironov, A.B. Effect of Fe on the structure of potassium borosilicate glasses. Inorg. Mater. 2015, 51, 177–181. [Google Scholar] [CrossRef]
- Eltabey, M.M.; Othman, H.A.; Ibrahim, S.E.; El-deen, L.M.S.; Elkholy, M.M. Structural, Electrical and Magnetic Properties of High Iron Content Sodium Borosilicate Glass. Analysis 2016, 8, 95–102. [Google Scholar]
- Meikhail, M.S.; Abdelghany, A.M. Structure and Electrical Properties of Iron Borosilicate Glasses. Silicon 2017, 9, 895–900. [Google Scholar] [CrossRef]
- Möncke, D.; Ehrt, D.; Varsamis, C.P.E.; Kamitsos, E.I.; Kalampounias, A.G. Thermal history of a low alkali borosilicate glass probed by infrared and Raman spectroscopy. Glass Technol. -Eur. J. Glass Sci. Technol. Part A 2006, 47, 133–137. [Google Scholar]
- Husung, R.D.; Doremus, R.H. The infrared transmission spectra of four silicate glasses before and after exposure to water. J. Mater. Res. 1990, 5, 2209–2217. [Google Scholar] [CrossRef]
- Brown, L.D. Characterisation of Australian Opals. Ph.D. Thesis, University of Technology Sydney, Ultimo, Australia, 2005. [Google Scholar]
- Clarke, T.A.; Rizkalla, E.N. X-ray photoelectron spectroscopy of some silicates. Chem. Phys. Lett. 1976, 37, 523–526. [Google Scholar] [CrossRef]
- Miura, Y.; Kusano, H.; Nanba, T.; Matsumoto, S. X-ray photoelectron spectroscopy of sodium borosilicate glasses. J. Non. Cryst. Solids 2001, 290, 1–14. [Google Scholar] [CrossRef]
- Briggs, D. X-ray Photoelectron Spectroscopy (XPS). In Handbook of Adhesion, 2nd ed.; Wiley: Hoboken, NJ, USA, 2005; pp. 621–622. [Google Scholar] [CrossRef]
- Zhang, W.; Song, S.; Nath, M.; Xue, Z.; Ma, G.; Li, Y. Inhibition of Cr6+ by the formation of in-situ Cr3+ containing solid-solution in Al2O3–CaO–Cr2O3–SiO2 system. Ceram. Int. 2021, 47, 9578–9584. [Google Scholar] [CrossRef]
- Mao, L.; Wang, J.; Zeng, M.; Zhang, W.; Hu, L.; Peng, M. Temperature dependent reduction of Cr(VI) to Cr(V) aroused by CaO during thermal treatment of solid waste containing Cr(VI). Chemosphere 2021, 262, 127924. [Google Scholar] [CrossRef] [PubMed]
- Nicoleau, E.; Schuller, S.; Angeli, F.; Charpentier, T.; Jollivet, P.; Le Gac, A.; Fournier, M.; Mesbah, A.; Vasconcelos, F. Phase separation and crystallization effects on the structure and durability of molybdenum borosilicate glass. J. Non. Cryst. Solids 2015, 427, 120–133. [Google Scholar] [CrossRef]
- Youngman, R.E. Borosilicate Glasses. Encycl. Glass Sci. Technol. Hist. Cult. 2021, II, 867–878. [Google Scholar] [CrossRef]
- De Jong, B.H.W.S.; Van Hoek, J.; Veeman, W.S.; Manson, D. V X-ray diffraction and 29Si magic-angle-spinning NMR of opals; incoherent long-and short-range order in opal-CT. Am. Mineral. 1987, 72, 1195–1203. [Google Scholar]
- Thomas, E.S.; Thompson, J.G.; Withers, R.L.; Sterns, M.; Xiao, Y.; Kirkpatrick, R.J. Further Investigation of the Stabilization of β-Cristobalite. J. Am. Ceram. Soc. 1994, 77, 49–56. [Google Scholar] [CrossRef]
- Mazurin, O.V.; Porai-Koshits, E.A. Phase Separation in Glass; Mazurin, O.V., Porai-Koshits, E.A., Eds.; Wiley: North-Holland, Amsterdam; Oxford, UK; New York, NY, USA; Tokyo, Japan, 1984. [Google Scholar]
- Konon, M.Y.; Stolyar, S.V.; Polyakova, I.G.; Drozdova, I.A.; Kurilenko, L.N. Phase separation in the glasses of the (8–x)Na2O · xFe2O3 · 22B2O3 · 70SiO2 system. Glass Phys. Chem. 2016, 42, 631–634. [Google Scholar] [CrossRef]
- Konon, M.; Antropova, T.; Kostyreva, T.; Drozdova, I.; Polyakova, I. Leaching of phase-separated glasses in Na2O-B2O3-SiO2-Fe2O3 system. Chem. Technol. 2016, 67, 7–12. [Google Scholar] [CrossRef]
- Wright, A.C.; Sinclair, R.N.; Shaw, J.L.; Haworth, R.; Bingham, P.A.; Forder, S.D.; Holland, D.; Scales, C.R.; Cuello, G.J.; Vedishcheva, N.M. The environment of Fe3+/Fe2+ cations in a sodium borosilicate glass. Phys. Chem. Glass Eur. J. Glass Sci. Technol. Part B 2017, 58, 78–91. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Wu, T. Effect of Cr2O3 on the crystallization behavior of synthetic diopside and characterization of Cr-doped diopside glass ceramics. Ceram. Int. 2018, 44, 10119–10129. [Google Scholar] [CrossRef]







| Glass Designation | Binding Energy (eV) (FWHM (eV)) | ||||||
|---|---|---|---|---|---|---|---|
| O1s BO 1s | O1s NBO 1s | Na1s | B1s | Si2p | Cr2p | O1s MeO | |
| Annealed | 532.80 (1.82) | 531.98 (1.90) | 1072.17 (2.20) | 193.30 (1.99) | 103.42 (1.78) | 576.50 (3.76) | 530.05 (1.60) |
| 550 °C, 24 h | 532.78 (1.79) | 531.89 (1.72) | 1072.10 (2.16) | 193.09 (1.97) | 103.38 (1.83) | 576.65 (3.93) | 530.22 (1.69) |
| 550 °C, 96 h | 532.71 (1.81) | 531.86 (1.78) | 1071.89 (2.07) | 192.99 (1.88) | 103.31 (1.79) | 576.51 (3.43) | 530.28 (1.70) |
| Percentage, % | Quenched | Annealed | 550 °C, 24 h | 550 °C, 96 h |
|---|---|---|---|---|
| Boron units | ||||
| BO3 ring | 20.33 | 32.91 | 32.34 | 35.46 |
| BO3 non-ring | 36.38 | 32.91 | 32.34 | 35.46 |
| BO4(3Si, 1B) | 38.22 | 34.17 | 35.32 | 29.08 |
| BO4(4Si) | 5.07 | - | - | - |
| Silicon units | ||||
| Q4 (3Si,1BO4) | 23 | 34 | 52 | 44 |
| Q4(3Si, 1BO3) | 73 | 66 | 44 | 29 |
| Q4(4Si) | 4 | - | 4 | 27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konon, M.; Polyakova, I.G.; Mazur, A.S.; Saratovskii, A.S.; Danilovich, D.P.; Alikin, M. Crystallization of Cristobalite in Sodium Borosilicate Glass in the Presence of Cr2O3. Materials 2023, 16, 5016. https://doi.org/10.3390/ma16145016
Konon M, Polyakova IG, Mazur AS, Saratovskii AS, Danilovich DP, Alikin M. Crystallization of Cristobalite in Sodium Borosilicate Glass in the Presence of Cr2O3. Materials. 2023; 16(14):5016. https://doi.org/10.3390/ma16145016
Chicago/Turabian StyleKonon, Marina, Irina G. Polyakova, Anton S. Mazur, Artem S. Saratovskii, Dmitry P. Danilovich, and Mikhail Alikin. 2023. "Crystallization of Cristobalite in Sodium Borosilicate Glass in the Presence of Cr2O3" Materials 16, no. 14: 5016. https://doi.org/10.3390/ma16145016