3.1. Carbon-Free Alloys
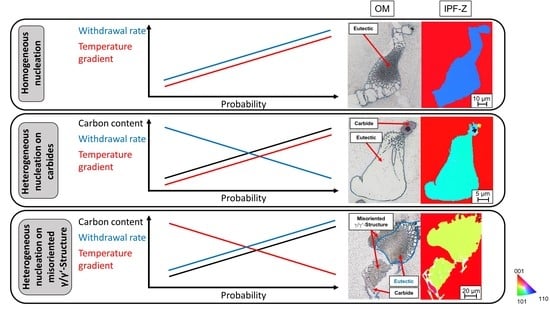
In carbon-free alloys, micro-stray grains with a slightly different crystallographic orientation than the single crystal were formed in the interdendritic region during single-crystal solidification (
Figure 2a,c). As illustrated in
Table 4, they were only formed in the alloy CMSX-4. Since the lattice structure of the micro-stray grains was observed to be identical to that of the eutectics, they represented a preferred nucleating surface for the eutectics. The difference of the micro stray-grain compared with the eutectic was the morphology, as seen in
Figure 2a,c,e,g.
In the carbon-free alloys, misoriented eutectics were also found in the interdendritic region due to homogeneous nucleation from the residual melt in the interdendritic regions (
Figure 2b,d), which was observed in both CMSX-4 and CMSX-6. The phenomenon in the alloy CMSX-4, however, only occurred at high-temperature gradients of 3.3 K/mm and high withdrawal rates starting from 4 mm/min, as listed in
Table 5. In CMSX-6, homogeneous eutectics were detected at all process parameters applied in this study.
In the Bridgman–Stockbarger process, it was noticeable that no homogeneous eutectics were formed in the alloy CMSX-4. In the Bridgman process, on the other hand, this effect was only seen up to two times per sample cross-section for the alloy CMSX-4, whereas plenty was contained in the CMSX-6 alloy. All in all, the eutectics were preferentially formed through homogeneous nucleation from the residual melt at higher cooling rates and in the CMSX-6 rather than in the CMSX-4 alloy.
3.2. Carbon-Containing Alloys
In carbon-containing nickel-based superalloys, carbides with different morphologies were formed in the interdendritic regions. As demonstrated in
Figure 3 for alloys CMSX-6-LC1 and CMSX-6-LC2, the carbide core exhibited an octahedral shape, whereby the arms extended from the corners of the octahedra. The influence of withdrawal rates on the formation affinity of the arms can also be seen in
Figure 3. For example, in the Bridgman–Stockbarger process, the arms appeared in the alloys only from a withdrawal rate of ≥1 mm/min. The ends of the arms were arrowhead-shaped (
Figure 3e). As shown in
Figure 3c,f, plates could also form between the arms at higher withdrawal rates. Furthermore, it was visible that the size of the octahedra decreased with increasing withdrawal rates and the branching of arms formed more complex structures at the withdrawal rate of 4 mm/min. At the withdrawal rate of 0.25 mm/min, no arms were formed; instead, several layers were formed around the octahedra (
Figure 3a,d). Analogously, these formation mechanisms were also observed in the Bridgman experiments (
Figure 4).
Similar carbide morphologies were also observed in the CM-247-LC and MAR-M-247 alloys. However, the carbides grew into larger carbides due to the higher carbon content compared with the CMSX-6 series. Nevertheless, the origin consisted of an octahedral core having extended dendrite-like arms at the corners. Due to the higher carbon content, large branched morphologies were formed, which could grow together and form closed chambers due to the plate formation between the arms (
Figure 3g). At higher temperature gradients, as in the Bridgman–Stockbarger process, the carbides became smaller and more compact than those in the industrial Bridgman system. Furthermore, the carbide size decreased with increasing withdrawal rates. The carbides were limited in size by the dendrites and adapted in shape to the dendrites at the end of solidification.
The formation of complex intensely branched carbide arms with plates (e.g.,
Figure 3g) resulted in a closed-chamber structure which could entrap the residual melt. The residual melt then solidified differently from the single crystal. However, the chambers may not have been completely closed, but open in one or more directions. In which direction a carbide structure was closed and open was random, since the carbide could move and rotate freely at the early stage of growth if there was enough interdendritic space. From these results, it could be concluded that, if the carbide structure was open in the direction opposite to the growth direction, the single crystal could produce a small orientation deviation while growing into the residual melt entrapped in the carbide (
Figure 5a,e,j,o). Furthermore, the entrapped melt could nucleate at the carbide surface when the carbide was completely closed against the growth direction. As a result, an identical crystallographic orientation of the carbide and the solidified entrapped melt was formed (
Figure 5f,k,p). The different enclosed chambers in a carbide could also have slightly different orientations, as illustrated in the upper part of
Figure 5k. The carbide chamber with the micro-stray grain could also be open in the solidification direction, causing the misorientation to grow into the interdendritic region, which is shown in the EBSD measurement in
Figure 5b,g,l,q. Moreover, eutectics could also nucleate at micro-stray grains that had been formed in a carbide and grown into the residual melt, as shown in
Figure 5b,g,l,q. This was based on the very similar lattice defect between the γ-phase and the eutectics, identical to the homogeneously solidified micro-stray grain in the CMSX-4 alloy (
Section 3.1).
The mechanism of entrapping the residual melt in carbides was not detected at low carbon content, as in the CMSX-6-LC1, and increased with the addition of carbon (
Table 6). The temperature gradient also influenced this effect, i.e., in the Bridgman–Stockbarger process with higher temperature gradients, this effect only occurred at higher carbon contents compared with the Bridgman process with lower temperature gradients. Furthermore, it could also be seen that this effect occurred preferably at higher withdrawal rates, since it did not occur in CM-247-LC W1 but occurred in the same alloy at a higher withdrawal rate of W4.
Eutectics could also nucleate directly on carbides and consequently adopt the crystallographic orientation of the corresponding carbide. This effect was observed both on blocky carbides in the alloy CMSX-6-LC2 (
Figure 5c,h,m,r) and on Chinese-script-shaped ones in MAR-M-247 (
Figure 5d,i,n,s). Stepwise grinding ensured that these eutectics were not nucleated on micro-stray grains as described previously.
The conditions in which eutectic nucleation was detected on a carbide are listed in
Table 7. In the Bridgman process, it was shown that no nucleation of eutectics on carbides was detected at low carbon contents, such as CMSX-6-LC1, or at very high withdrawal rates, such as 5.5 mm/min. This phenomenon was also seen in the Bridgman–Stockbarger process with higher temperature gradients. However, the nucleation shifted to lower withdrawal rates and higher carbon contents. Consequently, in the Bridgman–Stockbarger process, the nucleation of eutectics on carbides was also no longer possible for the alloy CMSX-6-LC2 above a withdrawal rate of 1 mm/min. This nucleation mechanism was also observed in MAR-M-247 only up to Q-W1.
The number of eutectics nucleated on carbides per area was analyzed using mosaic micrographs over the entire sample cross-section of CMSX-6-LC2 and MAR-M-247 at Q-W0.25. In CMSX-6-LC2 Q-W0.25, one eutectic nucleated on a carbide per six mm2 was detected, while five eutectics were detected in MAR-M-247 Q-W0.25 in the same area. In addition, the sample MAR-M-247 Q-W1 was investigated and three misoriented eutectics were found in the same area (three misoriented eutectics per six mm2). In summary, the number of eutectics nucleated on carbides per area increased with increasing carbon contents and decreased with increasing withdrawal rates.