Thermal Expansion and Thermal Conductivity of Ni/Graphene Composite: Molecular Dynamics Simulation
Abstract
:1. Introduction
2. Simulation Details
2.1. Composite Structure
2.2. Interatomic Potentials
2.3. Calculation of Thermal Expansion Coefficient
2.4. Calculation of the Thermal Conductivity
3. Results
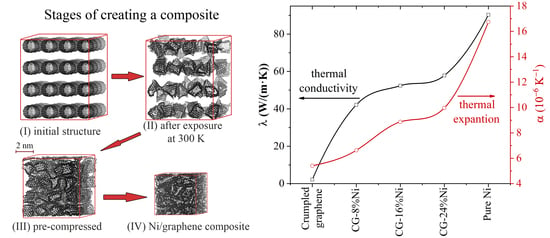
3.1. Thermal Expansion Coefficient
3.2. Thermal Conductivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MD | Molecular Dynamics |
| TEC | Thermal Expansion Coefficient |
| TCC | Thermal Conductivity Coefficient |
| CG | Crumpled Graphene |
References
- Han, R.; Song, H.; An, M. Atomic simulation of interaction mechanism between dislocation and graphene in graphene/aluminum composites. Comput. Mater. Sci. 2021, 197, 110604. [Google Scholar] [CrossRef]
- Zhan, K.; Zhao, R.; Li, F.; Wang, T.; Mo, W.; Yang, Z.; Zhao, B. Fabrication of graphite/Cu composite foils with ultrahigh thermal conductivity by adding an intermediate nickel layer and vacuum hot pressing treatment. J. Alloys Compd. 2021, 886, 161228. [Google Scholar] [CrossRef]
- Cao, L.; Wang, C.; Huang, Y. Structure optimization of graphene aerogel-based composites and applications in batteries and supercapacitors. Chem. Eng. J. 2023, 454, 140094. [Google Scholar] [CrossRef]
- Zhang, T.; Gao, X.; Li, J.; Xiao, L.; Gao, H.; Zhao, F.; Ma, H. Progress on the application of graphene-based composites toward energetic materials: A review. Def. Technol. 2023; in press. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Q.; Wang, S. A review on enhancement of mechanical and tribological properties of polymer composites reinforced by carbon nanotubes and graphene sheet: Molecular dynamics simulations. Compos. Part B Eng. 2019, 160, 348–361. [Google Scholar] [CrossRef]
- Sun, J.; Bai, Z.; Huang, Z.; Zhang, Z. Preparation of CdSe/RGO-GO composites with the high performance of adsorption and degradation. Lett. Mater. 2020, 10, 200–205. [Google Scholar] [CrossRef]
- Glukhova, O.; Mitrofanov, V.; Slepchenkov, M. The effect of the occurrence of a magnetic field of a current loop in hybrid graphen/C60 carbon systems. Lett. Mater. 2020, 10, 491–495. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, K.; Liu, G.; Chen, Y.; Wang, M.; Li, S.; Li, R. Recent advances on graphene: Synthesis, properties and applications. Compos. Part A Appl. Sci. Manuf. 2022, 160, 107051. [Google Scholar] [CrossRef]
- Deepa, C.; Rajeshkumar, L.; Ramesh, M. Preparation, synthesis, properties and characterization of graphene-based 2D nano-materials for biosensors and bioelectronics. J. Mater. Res. Technol. 2022, 19, 2657–2694. [Google Scholar] [CrossRef]
- Wang, P.; Liu, W.; Chen, L.; Mu, C.; Qi, G.; Bian, F. Bio-inspired laminated graphite nanosheets/copper composites. RSC Adv. 2015, 5, 51342–51346. [Google Scholar] [CrossRef]
- Ashwath, P.; Jeyapandiarajan, P.; Joel, J.; Kumar, H.P.; Xavior, M.A.; Sumanth, N.; Reddy, C.S.; Deepa. Flexural studies of graphene reinforced aluminium metal matrix composite. Mater. Today Proc. 2018, 5, 13459–13463. [Google Scholar] [CrossRef]
- Ashwath, P.; Joel, J.; Kumar, H.P.; Xavior, M.A.; Goel, A.; Nigam, T.; Rathi, M. Processing and characterization of extruded 2024 series of aluminum alloy. Mater. Today Proc. 2018, 5, 12479–12483. [Google Scholar] [CrossRef]
- Fu, K.; Zhang, X.; Shi, C.; Liu, E.; He, F.; Li, J.; Zhao, N.; He, C. An approach for fabricating Ni@graphene reinforced nickel matrix composites with enhanced mechanical properties. Mater. Sci. Eng. A 2018, 715, 108–116. [Google Scholar] [CrossRef]
- Sharma, A.; Vasudevan, B.; Sujith, R.; Kotkunde, N.; Suresh, K.; Gupta, A.K. Effect of Graphene Nanoplatelets on the Mechanical Properties of Aluminium Metal Matrix Composite. Mater. Today Proc. 2019, 18, 2461–2467. [Google Scholar] [CrossRef]
- Xie, Z.; Guo, H.; Zhang, Z.; Zhang, X. Thermal expansion behaviour and dimensional stability of Diamond/Cu composites with different diamond content. J. Alloys Compd. 2019, 797, 122–130. [Google Scholar] [CrossRef]
- Ostovari, F.; Hasanpoori, M.; Abbasnejad, M.; Salehi, M.A. DFT calculations of graphene monolayer in presence of Fe dopant and vacancy. Phys. B Condens. Matter 2018, 541, 6–13. [Google Scholar] [CrossRef]
- Kamedulski, P.; Kaczmarek-Kedziera, A.; Lukaszewicz, J.P. Influence of intermolecular interactions on the properties of carbon nanotubes. Bull. Mater. Sci. 2018, 41, 76. [Google Scholar] [CrossRef] [Green Version]
- Gecim, G.; Ozekmekci, M. A density functional theory study of molecular H2S adsorption on (4,0) SWCNT doped with Ge, Ga and B. Surf. Sci. 2021, 711, 121876. [Google Scholar] [CrossRef]
- Kumar, P.L.; Lombardi, A.; Byczynski, G.; Murty, S.N.; Murty, B.; Bichler, L. Recent advances in aluminium matrix composites reinforced with graphene-based nanomaterial: A critical review. Prog. Mater. Sci. 2022, 128, 100948. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, B.; Ma, L.; Liu, G.; Qian, S.; Xu, Z.; Liu, E.; Zhang, X.; He, C.; Zhao, N. Architectured interfacial interlocking structure for enhancing mechanical properties of Al matrix composites reinforced with graphene nanosheets. Carbon 2021, 183, 685–701. [Google Scholar] [CrossRef]
- Xu, L.; Wang, R.; Gen, M.; Lu, L.; Han, G. Preparation and Properties of Graphene/Nickel Composite Coating Based on Textured Surface of Aluminum Alloy. Materials 2019, 12, 3240. [Google Scholar] [CrossRef] [Green Version]
- Luo, F.; Jiang, X.; Sun, H.; Mo, D.; Zhang, Y.; Shu, R.; Xue, L. Microstructures, mechanical and thermal properties of diamonds and graphene hybrid reinforced laminated Cu matrix composites by vacuum hot pressing. Vacuum 2023, 207, 111610. [Google Scholar] [CrossRef]
- Park, C.W.; Lee, J.H.; Seo, J.K.; Jo, W.Y.; Whang, D.; Hwang, S.M.; Kim, Y.J. Graphene collage on Ni-rich layered oxide cathodes for advanced lithium-ion batteries. Nat. Commun. 2021, 12, 2145. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Calizo, I.; Teweldebrhan, D.; Pokatilov, E.P.; Nika, D.L.; Balandin, A.A.; Bao, W.; Miao, F.; Lau, C.N. Extremely high thermal conductivity of graphene: Prospects for thermal management applications in nanoelectronic circuits. Appl. Phys. Lett. 2008, 92, 151911. [Google Scholar] [CrossRef] [Green Version]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior Thermal Conductivity of Single-Layer Graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Jaćimovski, S.K.; Bukurov, M.; Šetrajčić, J.P.; Raković, D.I. Phonon thermal conductivity of graphene. Superlattices Microstruct. 2015, 88, 330–337. [Google Scholar] [CrossRef]
- Ivakin, E.V.; Sukhodolov, A.V.; Ralchenko, V.G.; Vlasov, A.V.; Khomich, A.V. Measurement of thermal conductivity of polycrystalline CVD diamond by laser-induced transient grating technique. Quantum Electron. 2002, 32, 367–372. [Google Scholar] [CrossRef]
- Fugallo, G.; Cepellotti, A.; Paulatto, L.; Lazzeri, M.; Marzari, N.; Mauri, F. Thermal Conductivity of Graphene and Graphite: Collective Excitations and Mean Free Paths. Nano Lett. 2014, 14, 6109–6114. [Google Scholar] [CrossRef]
- Zhao, L.; Tang, J.; Zhou, M.; Shen, K. A review of the coefficient of thermal expansion and thermal conductivity of graphite. New Carbon Mater. 2022, 37, 544–555. [Google Scholar] [CrossRef]
- Kim, P.; Shi, L.; Majumdar, A.; McEuen, P.L. Thermal Transport Measurements of Individual Multiwalled Nanotubes. Phys. Rev. Lett. 2001, 87, 215502. [Google Scholar] [CrossRef] [Green Version]
- Pop, E.; Mann, D.; Wang, Q.; Goodson, K.; Dai, H. Thermal Conductance of an Individual Single-Wall Carbon Nanotube above Room Temperature. Nano Lett. 2005, 6, 96–100. [Google Scholar] [CrossRef] [Green Version]
- Malekpour, H.; Ramnani, P.; Srinivasan, S.; Balasubramanian, G.; Nika, D.L.; Mulchandani, A.; Lake, R.K.; Balandin, A.A. Thermal conductivity of graphene with defects induced by electron beam irradiation. Nanoscale 2016, 8, 14608–14616. [Google Scholar] [CrossRef] [Green Version]
- Nika, D.L.; Ghosh, S.; Pokatilov, E.P.; Balandin, A.A. Lattice thermal conductivity of graphene flakes: Comparison with bulk graphite. Appl. Phys. Lett. 2009, 94, 203103. [Google Scholar] [CrossRef] [Green Version]
- Zakharchenko, K.V.; Katsnelson, M.I.; Fasolino, A. Finite Temperature Lattice Properties of Graphene beyond the Quasiharmonic Approximation. Phys. Rev. Lett. 2009, 102, 046808. [Google Scholar] [CrossRef]
- Zakharchenko, K.V.; Los, J.H.; Katsnelson, M.I.; Fasolino, A. Atomistic simulations of structural and thermodynamic properties of bilayer graphene. Phys. Rev. B 2010, 81, 235439. [Google Scholar] [CrossRef] [Green Version]
- Yoon, D.; Son, Y.W.; Cheong, H. Strain-Dependent Splitting of the Double-Resonance Raman Scattering Band in Graphene. Phys. Rev. Lett. 2011, 106, 155502. [Google Scholar] [CrossRef] [Green Version]
- Mounet, N.; Marzari, N. First-principles determination of the structural, vibrational and thermodynamic properties of diamond, graphite, and derivatives. Phys. Rev. B 2005, 71, 205214. [Google Scholar] [CrossRef] [Green Version]
- Bao, W.; Miao, F.; Chen, Z.; Zhang, H.; Jang, W.; Dames, C.; Lau, C.N. Controlled ripple texturing of suspended graphene and ultrathin graphite membranes. Nat. Nanotechnol. 2009, 4, 562–566. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.Z.; Mahboob, M.; Lowe, L.R.; Bechtel, E.S. Characterization of the thermal expansion properties of graphene using molecular dynamics simulations. J. Phys. D Appl. Phys. 2013, 46, 435302. [Google Scholar] [CrossRef]
- Si, Y.; Samulski, E.T. Exfoliated Graphene Separated by Platinum Nanoparticles. Chem. Mater. 2008, 20, 6792–6797. [Google Scholar] [CrossRef]
- Wei, N.; Zhou, C.; Li, Z.; Ou, B.; Zhao, K.; Yu, P.; Li, S.; Zhao, J. Thermal conductivity of Aluminum/Graphene metal-matrix composites: From the thermal boundary conductance to thermal regulation. Mater. Today Commun. 2022, 30, 103147. [Google Scholar] [CrossRef]
- Ruch, P.; Beffort, O.; Kleiner, S.; Weber, L.; Uggowitzer, P. Selective interfacial bonding in Al(Si)–diamond composites and its effect on thermal conductivity. Compos. Sci. Technol. 2006, 66, 2677–2685. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, H.; Zhang, Y.; Li, J.; Wang, X. Effect of copper content on the thermal conductivity and thermal expansion of Al–Cu/diamond composites. Mater. Des. 2012, 39, 87–92. [Google Scholar] [CrossRef]
- An, Z.; Li, J.; Kikuchi, A.; Wang, Z.; Jiang, Y.; Ono, T. Mechanically strengthened graphene-Cu composite with reduced thermal expansion towards interconnect applications. Microsyst. Nanoeng. 2019, 5, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, J.; Niu, L.; Qiao, Y.; Chen, P.; Rittel, D. Impact energy absorption behavior of graphene aerogels prepared by different drying methods. Mater. Des. 2022, 221, 110912. [Google Scholar] [CrossRef]
- Zhu, Z.; Sun, X.; Li, G.; Xue, H.; Guo, H.; Fan, X.; Pan, X.; He, J. Microwave-assisted synthesis of graphene–Ni composites with enhanced microwave absorption properties in Ku-band. J. Magn. Magn. Mater. 2015, 377, 95–103. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, M.; Du, J.; Zhang, W.; Zhou, S.; Ren, W.; Zhou, Q.; Shi, L. Construction of graphene network in Ni matrix composites: A molecular dynamics study of densification process. Carbon 2022, 191, 55–66. [Google Scholar] [CrossRef]
- Safina, L.R.; Krylova, K.A.; Baimova, J.A. Molecular dynamics study of the mechanical properties and deformation behavior of graphene/metal composites. Mater. Today Phys. 2022, 28, 100851. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Liu, H.; Zhang, L.; Zhang, Y.; Xu, C.; Gong, C. Biomass-derived heterogeneous RGO/Ni/C composite with hollow structure for high-efficiency electromagnetic wave absorption. Mater. Today Phys. 2023, 31, 100966. [Google Scholar] [CrossRef]
- Safina, L.R.; Baimova, J.A.; Mulyukov, R.R. Nickel nanoparticles inside carbon nanostructures: Atomistic simulation. Mech. Adv. Mater. Mod. Process. 2019, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.; Hu, D.; Wang, X.; Zhang, H.; Du, P. Carbon layer coated nickel phosphide nanoparticles embedded in three-dimensional graphene network for high-performance supercapacitor. J. Energy Storage 2022, 50, 104713. [Google Scholar] [CrossRef]
- Geramilla, M.; Muthukumaravel, C.; Karunakaran, U.; Sairam, T. Gold nanoparticle decorated vertical graphene nanosheets composite/hybrid for acetone sensing at room temperature. Mater. Sci. Eng. B 2023, 288, 116211. [Google Scholar] [CrossRef]
- Vardanyan, V.H.; Urbassek, H.M. Morphology of graphene flakes in Ni-graphene nanocomposites and its influence on hardness: An atomistic study. Carbon 2021, 185, 660–668. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Thompson, A.P.; Aktulga, H.M.; Berger, R.; Bolintineanu, D.S.; Brown, W.M.; Crozier, P.S.; in’t Veld, P.J.; Kohlmeyer, A.; Moore, S.G.; Nguyen, T.D.; et al. LAMMPS—A flexible simulation tool for particle-based materials modeling at the atomic, meso, and continuum scales. Comput. Phys. Commun. 2022, 271, 108171. [Google Scholar] [CrossRef]
- Bhalla, P.; Kumar, P.; Das, N.; Singh, N. Theory of the dynamical thermal conductivity of metals. Phys. Rev. B 2016, 94, 115114. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Gu, M.; Wang, Y.; Xu, L.; Tang, Y.; Wu, R. In-situ growth of Ni nanoparticle-encapsulated N-doped carbon nanotubes on carbon nanorods for efficient hydrogen evolution electrocatalysis. Nano Res. 2020, 13, 975–982. [Google Scholar] [CrossRef]
- Ding, L.P.; McLean, B.; Xu, Z.; Kong, X.; Hedman, D.; Qiu, L.; Page, A.J.; Ding, F. Why Carbon Nanotubes Grow. J. Am. Chem. Soc. 2022, 144, 5606–5613. [Google Scholar] [CrossRef] [PubMed]
- Ganz, E.; Ganz, A.B.; Yang, L.M.; Dornfeld, M. The initial stages of melting of graphene between 4000 K and 6000 K. Phys. Chem. Chem. Phys. 2017, 19, 3756–3762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Heim, F.M.; Bartlett, J.L.; Song, N.; Isheim, D.; Li, X. Bioinspired, graphene-enabled Ni composites with high strength and toughness. Sci. Adv. 2019, 5, eaav5577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krylova, K.; Baimova, J.; Mulyukov, R. Effect of deformation on dehydrogenation mechanisms of crumpled graphene: Molecular dynamics simulation. Lett. Mater. 2019, 9, 81–85. [Google Scholar] [CrossRef]
- Safina, L.; Baimova, J.; Krylova, K.; Murzaev, R.; Mulyukov, R. Simulation of metal-graphene composites by molecular dynamics: A review. Lett. Mater. 2020, 10, 351–360. [Google Scholar] [CrossRef]
- Safina, L.R.; Baimova, J.A.; Krylova, K.A.; Murzaev, R.T.; Shcherbinin, S.A.; Mulyukov, R.R. Ni–Graphene Composite Obtained by Pressure–Temperature Treatment: Atomistic Simulations. Phys. Status Solidi (RRL)–Rapid Res. Lett. 2021, 15, 2100429. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Mendelev, M.; Kramer, M.; Hao, S.; Ho, K.; Wang, C. Development of interatomic potentials appropriate for simulation of liquid and glass properties of NiZr2 alloy. Philos. Mag. 2012, 92, 4454–4469. [Google Scholar] [CrossRef]
- Stuart, S.J.; Tutein, A.B.; Harrison, J.A. A reactive potential for hydrocarbons with intermolecular interactions. J. Chem. Phys. 2000, 112, 6472–6486. [Google Scholar] [CrossRef] [Green Version]
- Miyazawa, K.; Tanaka, Y. LAMMPS molecular dynamics simulation of methane decomposition on nickel thin films at high temperatures. Surf. Sci. 2021, 713, 121904. [Google Scholar] [CrossRef]
- Polyakova, P.; Shcherbinin, S.; Baimova, J.A. Molecular dynamics investigation of atomic mixing and mechanical properties of Al/Ti interface. Lett. Mater. 2021, 11, 561–565. [Google Scholar] [CrossRef]
- Garg, M.; Ghosh, S.; Padmanabhan, V. Room temperature hydrogen storage in defective single-walled carbon nanotubes: A molecular dynamics study. Lett. Mater. 2021, 11, 321–326. [Google Scholar] [CrossRef]
- Apkadirova, N.; Krylova, K.; Mulyukov, R. Dehydrogenation of a Crumpled Graphene Flake: Molecular Dynamics. Mech. Phys. Mater. 2021, 47, 170. [Google Scholar] [CrossRef]
- Katin, K.P.; Prudkovskiy, V.S.; Maslov, M.M. Molecular dynamics simulation of nickel-coated graphene bending. Micro Nano Lett. 2018, 13, 160–164. [Google Scholar] [CrossRef]
- Galashev, A.Y.; Katin, K.P.; Maslov, M.M. Morse parameters for the interaction of metals with graphene and silicene. Phys. Lett. A 2019, 383, 252–258. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Pathak, V.K.; Singh, R.; Dikshit, M.K. Stress-strain behaviour of graphene reinforced aluminum nanocomposite under compressive loading using molecular dynamics. Mater. Today Proc. 2021, 44, 4521–4525. [Google Scholar] [CrossRef]
- Faria, B.; Silvestre, N.; Lopes, J.N. Strength and fracture of graphyne and graphdiyne nanotubes. Comput. Mater. Sci. 2020, 171, 109233. [Google Scholar] [CrossRef]
- Baimova, J.; Rysaeva, L.; Rudskoy, A. Deformation behavior of diamond-like phases: Molecular dynamics simulation. Diam. Relat. Mater. 2018, 81, 154–160. [Google Scholar] [CrossRef]
- Ghasemi, H.; Rajabpour, A. Thermal expansion coefficient of graphene using molecular dynamics simulation: A comparative study on potential functions. J. Phys. Conf. Ser. 2017, 785, 012006. [Google Scholar] [CrossRef] [Green Version]
- Pishkenari, H.N.; Mohagheghian, E.; Rasouli, A. Molecular dynamics study of the thermal expansion coefficient of silicon. Phys. Lett. A 2016, 380, 4039–4043. [Google Scholar] [CrossRef]
- Liu, B.; Reddy, C.D.; Jiang, J.; Zhu, H.; Baimova, J.A.; Dmitriev, S.V.; Zhou, K. Thermal conductivity of silicene nanosheets and the effect of isotopic doping. J. Phys. D Appl. Phys. 2014, 47, 165301. [Google Scholar] [CrossRef]
- Liu, B.; Baimova, J.A.; Reddy, C.D.; Dmitriev, S.V.; Law, W.K.; Feng, X.Q.; Zhou, K. Interface thermal conductance and rectification in hybrid graphene/silicene monolayer. Carbon 2014, 79, 236–244. [Google Scholar] [CrossRef]
- Zolotarevskiy, V.; Savin, A.V.; Gendelman, O.V. Heat conduction in a chain of dissociating particles: Effect of dimensionality. Phys. Rev. E 2015, 91, 032127. [Google Scholar] [CrossRef] [Green Version]
- Gavrilov, S.N.; Krivtsov, A.M. Steady-state ballistic thermal transport associated with transversal motions in a damped graphene lattice subjected to a point heat source. Contin. Mech. Thermodyn. 2021, 34, 297–319. [Google Scholar] [CrossRef]
- Kuzkin, V.A.; Krivtsov, A.M. Unsteady ballistic heat transport: Linking lattice dynamics and kinetic theory. Acta Mech. 2021, 232, 1983–1996. [Google Scholar] [CrossRef]
- Hafskjold, B.; Ikeshoji, T. Partial specific quantities computed by nonequilibrium molecular dynamics. Fluid Phase Equilibria 1995, 104, 173–184. [Google Scholar] [CrossRef]
- Wirnsberger, P.; Frenkel, D.; Dellago, C. An enhanced version of the heat exchange algorithm with excellent energy conservation properties. J. Chem. Phys. 2015, 143, 124104. [Google Scholar] [CrossRef] [Green Version]
- Müller-Plathe, F. A simple nonequilibrium molecular dynamics method for calculating the thermal conductivity. J. Chem. Phys. 1997, 106, 6082–6085. [Google Scholar] [CrossRef]
- Ghaderi, H.; Ghasemi, A.; Rouhi, S.; Mahdavi, E. Evaluation of the thermal conductivity coefficient of the strained concentric multi-walled carbon and boron-nitride nanotubes: A molecular dynamics investigation. Phys. E Low-Dimens. Syst. Nanostruct. 2021, 134, 114830. [Google Scholar] [CrossRef]
- Wu, X.; Han, Q. Maximum thermal conductivity of multilayer graphene with periodic two-dimensional empty space. Int. J. Heat Mass Transf. 2022, 191, 122829. [Google Scholar] [CrossRef]
- Wei, A.; Li, Y.; Ren, W.; Ye, W. An interlayer/intralayer coupling mechanism for the thermal characteristics of polycrystalline few-layer graphene. Appl. Phys. Lett. 2019, 114, 021902. [Google Scholar] [CrossRef]
- Chang, S.W.; Nair, A.K.; Buehler, M.J. Geometry and temperature effects of the interfacial thermal conductance in copper– and nickel–graphene nanocomposites. J. Phys. Condens. Matter 2012, 24, 245301. [Google Scholar] [CrossRef] [Green Version]
- Yuan, S.P.; Jiang, P.X. Thermal Conductivity of Small Nickel Particles. Int. J. Thermophys. 2006, 27, 581–595. [Google Scholar] [CrossRef]
- Jeong, G.U.; Park, C.S.; Do, H.S.; Park, S.M.; Lee, B.J. Second nearest-neighbor modified embedded-atom method interatomic potentials for the Pd-M (M = Al, Co, Cu, Fe, Mo, Ni, Ti) binary systems. Calphad 2018, 62, 172–186. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, G.; Qian, P.; Su, Y. Element segregation and thermal stability of Ni–Pd nanoparticles. J. Mater. Sci. 2022, 57, 7384–7399. [Google Scholar] [CrossRef]
- Ho, D.T.; Park, H.S.; Kim, S.Y.; Schwingenschlögl, U. Graphene Origami with Highly Tunable Coefficient of Thermal Expansion. ACS Nano 2020, 14, 8969–8974. [Google Scholar] [CrossRef] [PubMed]
- Mostério, N.C.B.; Fonseca, A.F. Thermal expansion behavior of holes in graphene nanomeshes. Phys. Rev. B 2014, 89, 195437. [Google Scholar] [CrossRef]
- Forzani, L.; Ramos, C.A.; Brigneti, E.V.; Gennaro, A.M.; Koropecki, R.R. Negative thermal expansion of nanoporous anodic aluminum oxide membranes. Appl. Phys. Lett. 2019, 114, 111901. [Google Scholar] [CrossRef] [Green Version]
- Nix, F.C.; MacNair, D. The Thermal Expansion of Pure Metals: Copper, Gold, Aluminum, Nickel, and Iron. Phys. Rev. 1941, 60, 597–605. [Google Scholar] [CrossRef]
- Verkhovtsev, A.V.; Schramm, S.; Solov’yov, A.V. Molecular dynamics study of the stability of a carbon nanotube atop a catalytic nanoparticle. Eur. Phys. J. D 2014, 68, 246. [Google Scholar] [CrossRef]
- Wejrzanowski, T.; Grybczuk, M.; Chmielewski, M.; Pietrzak, K.; Kurzydlowski, K.; Strojny-Nedza, A. Thermal conductivity of metal-graphene composites. Mater. Des. 2016, 99, 163–173. [Google Scholar] [CrossRef]
- Zhou, K.; Liu, B. Molecular Dynamics Simulation; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Ho, C.Y.; Powell, R.W.; Liley, P.E. Thermal Conductivity of Selected Materials; Part 2; US Department of Commerce, National Bureau of Standards: Washington, DC, USA, 1968. [Google Scholar]
- Yuan, S.; Jiang, P. Thermal conductivity of nanoscale thin nickel films. Prog. Nat. Sci. 2005, 15, 922–929. [Google Scholar] [CrossRef]
- Yue, C.; Feng, J.; Feng, J.; Jiang, Y. Low-thermal-conductivity nitrogen-doped graphene aerogels for thermal insulation. RSC Adv. 2016, 6, 9396–9401. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhou, M.; Huang, F.; Lin, T.; Wan, D. Effect of graphene aerogel on thermal behavior of phase change materials for thermal management. Sol. Energy Mater. Sol. Cells 2013, 113, 195–200. [Google Scholar] [CrossRef]
- Fan, Z.; Tng, D.Z.Y.; Lim, C.X.T.; Liu, P.; Nguyen, S.T.; Xiao, P.; Marconnet, A.; Lim, C.Y.; Duong, H.M. Thermal and electrical properties of graphene/carbon nanotube aerogels. Colloids Surfaces A Physicochem. Eng. Asp. 2014, 445, 48–53. [Google Scholar] [CrossRef]
- Le-Zakharov, A.A.; Krivtsov, A.M.; Porubov, A.V. Relation between defects and crystalline thermal conduction. Contin. Mech. Thermodyn. 2019, 31, 1873–1881. [Google Scholar] [CrossRef]
- Savin, A.V.; Gendelman, O.V. Mechanical control of heat conductivity in molecular chains. Phys. Rev. E 2014, 89, 012134. [Google Scholar] [CrossRef]
- Fan, Z.; Marconnet, A.; Nguyen, S.T.; Lim, C.Y.; Duong, H.M. Effects of heat treatment on the thermal properties of highly nanoporous graphene aerogels using the infrared microscopy technique. Int. J. Heat Mass Transf. 2014, 76, 122–127. [Google Scholar] [CrossRef]
- Krylova, K.A.; Safina, L.R.; Shcherbinin, S.A.; Baimova, J.A. Methodologyfor Molecular Dynamics Simulation of Plastic Deformation of a Nickel/Graphene Composite. Materials 2022, 15, 4038. [Google Scholar] [CrossRef]
- Safina, L.R.; Rozhnova, E.A. Molecular Dynamics Simulation of the Deformation Behavior of the Graphene/Al Composite. J. Struct. Chem. 2023, 64, 240–252. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murzaev, R.T.; Krylova, K.A.; Baimova, J.A. Thermal Expansion and Thermal Conductivity of Ni/Graphene Composite: Molecular Dynamics Simulation. Materials 2023, 16, 3747. https://doi.org/10.3390/ma16103747
Murzaev RT, Krylova KA, Baimova JA. Thermal Expansion and Thermal Conductivity of Ni/Graphene Composite: Molecular Dynamics Simulation. Materials. 2023; 16(10):3747. https://doi.org/10.3390/ma16103747
Chicago/Turabian StyleMurzaev, Ramil T., Karina A. Krylova, and Julia A. Baimova. 2023. "Thermal Expansion and Thermal Conductivity of Ni/Graphene Composite: Molecular Dynamics Simulation" Materials 16, no. 10: 3747. https://doi.org/10.3390/ma16103747