Study of the Functionalities of a Biochar Electrode Combined with a Photoelectrochemical Cell
Abstract
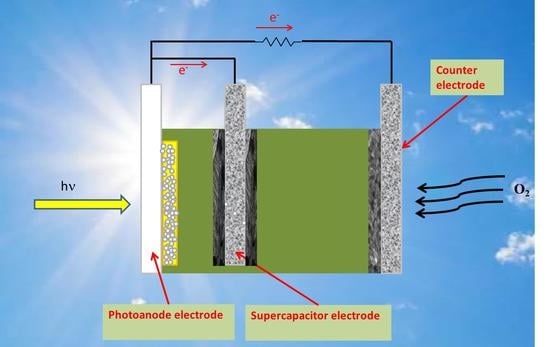
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Basic Characterization of the Biochar
3.2. Photoelectrochemical Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, Y.; Li, F.; Zhang, Q.; Liu, G.; Xue, C. Controllable Preparation of Green Biochar Based High-Performance Supercapacitors. Ionics 2022, 28, 2525–2561. [Google Scholar] [CrossRef]
- Gao, W.; Lin, Z.; Chen, H.; Yan, S.; Huang, Y.; Hu, X.; Zhang, S. A Review on N-Doped Biochar for Enhanced Water Treatment and Emerging Applications. Fuel Process. Technol. 2022, 237, 107468. [Google Scholar] [CrossRef]
- Al-Rumaihi, A.; Shahbaz, M.; Mckay, G.; Mackey, H.; Al-Ansari, T. A Review of Pyrolysis Technologies and Feedstock: A Blending Approach for Plastic and Biomass towards Optimum Biochar Yield. Renew. Sustain. Energy Rev. 2022, 167, 112715. [Google Scholar] [CrossRef]
- Mehdi, R.; Khoja, A.H.; Naqvi, S.R.; Gao, N.; Amin, N.A.S. A Review on Production and Surface Modifications of Biochar Materials via Biomass Pyrolysis Process for Supercapacitor Applications. Catalysts 2022, 12, 798. [Google Scholar] [CrossRef]
- Cheng, B.-H.; Zeng, R.J.; Jiang, H. Recent Developments of Post-Modification of Biochar for Electrochemical Energy Storage. Bioresour. Technol. 2017, 246, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Zheng, J.; Feng, L.; Du, C.; Jian, S.; Yang, W.; Wu, Y.A.; Jiang, S.; He, S.; Chen, W. Wood-Derived Biochar as Thick Electrodes for High-Rate Performance Supercapacitors. Biochar 2022, 4, 50. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.-P.; Qiu, L.-L.; Xiao, J.; Wu, F.-P.; Cao, J.-P.; Bai, Y.-H.; Liu, F.-J. Insights into the KOH Activation Parameters in the Preparation of Corncob-Based Microporous Carbon for High-Performance Supercapacitors. Diam. Relat. Mater. 2022, 129, 109331. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, J.; Yang, W. Green Supercapacitor Assisted Photocatalytic Fuel Cell System for Sustainable Hydrogen Production. Chem. Eng. J. 2021, 403, 126368. [Google Scholar] [CrossRef]
- Namsheer, K.; Chandra, S.R. Photo-powered integrated supercapacitors: A review on recent developments, challenges and future perspectives. J. Mater. Chem. A 2021, 9, 8248. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, X.; Yu, Z.; Yi, Y.; Huang, Z.; Lu, C. Preparation of High-Value Porous Carbon by Microwave Treatment of Chili Straw Pyrolysis Residue. Bioresour. Technol. 2022, 360, 127520. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, X.; Ma, R.; Sun, S.; Lin, J.; Luo, J.; Huang, H. Preparation of Hierarchical Porous Carbon by Pyrolyzing Sargassum under Microwave: The Internal Connection between Structure-oriented Regulation and Performance Optimization of Supercapacitors. J. Energy Storag. 2022, 53, 105190. [Google Scholar] [CrossRef]
- Dhawle, R.; Vakros, J.; Dracopoulos, V.; Manariotis, I.D.; Mantzavinos, D.; Lianos, P. Enhancement of the Photoelectrochemical Production of Hydrogen Peroxide under Intermittent Light Supply in the Presence of an Optimized Biochar Supercapacitor. Electrochim. Acta 2022, 427, 140846. [Google Scholar] [CrossRef]
- Giannakopoulos, S.; Vakros, J.; Dracopoulos, V.; Manariotis, I.D.; Mantzavinos, D.; Lianos, P. Enhancement of the Photoelectrocatalytic Degradation Rate of a Pollutant in the Presence of a Supercapacitor. J. Clean. Prod. 2022, 377, 134456. [Google Scholar] [CrossRef]
- Andrade, T.S.; Dracopoulos, V.; Lianos, P. Solar Energy Conversion and Storage Using a Photocatalytic Fuel Cell Combined with a Supercapacitor. Electronics 2021, 10, 273. [Google Scholar] [CrossRef]
- Andrade, T.S.; Vakros, J.; Mantzavinos, D.; Lianos, P. Biochar Obtained by Carbonization of Spent Coffee Grounds and Its Application in the Construction of an Energy Storage Device. Chem. Eng. J. Adv. 2020, 4, 100061. [Google Scholar] [CrossRef]
- Sfaelou, S.; Sygellou, L.; Dracopoulos, V.; Travlos, A.; Lianos, P. Effect of the Nature of Cadmium Salts on the Effectiveness of CdS SILAR Deposition and Its Consequences on the Performance of Sensitized Solar Cells. J. Phys. Chem. C 2014, 118, 22873–22880. [Google Scholar] [CrossRef]
- Santos Andrade, T.; Papagiannis, I.; Dracopoulos, V.; César Pereira, M.; Lianos, P. Visible-Light Activated Titania and Its Application to Photoelectrocatalytic Hydrogen Peroxide Production. Materials 2019, 12, 4238. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Williams, R.T. Physisorption Hysteresis Loops and the Characterization of Nanoporous Materials. Adsorpt. Sci. Technol. 2004, 22, 773–782. [Google Scholar] [CrossRef]
- Vakros, J.; Manariotis, I.D.; Dracopoulos, V.; Mantzavinos, D.; Lianos, P. Biochar from Spent Malt Rootlets and Its Application to an Energy Conversion and Storage Device. Chemosensors 2021, 9, 57. [Google Scholar] [CrossRef]
- Magioglou, E.; Frontistis, Z.; Vakros, J.; Manariotis, I.D.; Mantzavinos, D. Activation of Persulfate by Biochars from Valorized Olive Stones for the Degradation of Sulfamethoxazole. Catalysts 2019, 9, 419. [Google Scholar] [CrossRef] [Green Version]
- Ntaflou, M.; Vakros, J. Transesterification activity of modified biochars from spent malt rootlets using triacetin. J. Clean. Prod. 2020, 259, 120931. [Google Scholar] [CrossRef]
- Gao, G.; Cheong, L.Z.; Wang, D.; Shen, C. Pyrolytic carbon derived from spent cof- fee grounds as anode for sodium–ion batteries. Carbon Resour. Convers. 2018, 1, 104–108. [Google Scholar] [CrossRef]
- Grilla, E.; Vakros, J.; Konstantinou, I.; Manariotis, I.D.; Mantzavinos, D. Activation of persulfate by biochar from spent malt rootlets for the degradation of trimethoprim in the presence of inorganic ions. J. Chem. Technol. Biotechnol. 2020, 95, 2348–2358. [Google Scholar] [CrossRef]
- Lykoudi, A.; Frontistis, Z.; Vakros, J.; Manariotis, I.D.; Mantzavinos, D. Degradation of sulfamethoxazole with persulfate using spent coffee grounds biochar as activator. J. Environ. Manag. 2020, 271, 111022. [Google Scholar] [CrossRef]
- Avramiotis, E.; Frontistis, Z.; Manariotis, I.D.; Vakros, J.; Mantzavinos, D. Oxidation of Sulfamethoxazole by Rice Husk Biochar-Activated Persulfate. Catalysts 2021, 11, 850. [Google Scholar] [CrossRef]
- Bourikas, K.; Vakros, J.; Kordulis, C.; Lycourghiotis, A. Potentiometric Mass Titrations: Experimental and Theoretical Establishment of a New Technique for Determining the Point of Zero Charge (PZC) of Metal (Hydr)Oxides. J. Phys. Chem. B 2003, 107, 9441–9451. [Google Scholar] [CrossRef]
- Pop, L.-C.; Sfaelou, S.; Lianos, P. Cation Adsorption by Mesoporous Titania Photoanodes and Its Effect on the Current-Voltage Characteristics of Photoelectrochemical Cells. Electrochim. Acta 2015, 156, 223–227. [Google Scholar] [CrossRef]
- Ito, S.; Chen, P.; Comte, P.; Nazeeruddin, M.K.; Liska, P.; Pechy, P.; Gratzel, M. Fabrication of Screen-Printing Pastes From TiO2 Powders for Dye-Sensitised Solar Cells. Prog. Photovolt Res. Appl. 2007, 15, 603–612. [Google Scholar] [CrossRef]










| Sample | Preparation Method | SSA (m2 g−1) | Micropores SSA (m2 g−1) | Pore Volume (mL g−1) | Point of Zero Charge |
|---|---|---|---|---|---|
| Raw BC | Pyrolysis or raw biomass at 850 °C | 100 | 58 | 0.077 | 8.4 |
| Base-treated low temp. BC | Treatment of raw BC at 105 °C with 1M NaOH | 363 | 175 | 0.247 | 9.6 |
| Activated BC | Pyrolysis of raw BC at 850 °C mixed with KOH | 1148 | 690 | 0.99 | >10.5 if any |
| Element | % Atomic Concentration | ||
|---|---|---|---|
| Raw BC | Base Treated BC | Activated BC | |
| C | 84.2 | 83.2 | 88.0 |
| O | 10.3 | 11.1 | 10.2 |
| Na | N.D. | 1.7 | 0.7 |
| K | 2.4 | 0.7 | 0.3 |
| Mg | 0.2 | 0.6 | N.D. |
| Si | 1.0 | 0.3 | N.D. |
| P | 1.0 | 1.4 | N.D. |
| S | 0.2 | 0.2 | 0.8 |
| Cl | 0.5 | 0.1 | N.D. |
| Ca | 0.2 | 0.7 | N.D. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannakopoulos, S.; Vakros, J.; Manariotis, I.D.; Mantzavinos, D.; Lianos, P. Study of the Functionalities of a Biochar Electrode Combined with a Photoelectrochemical Cell. Materials 2023, 16, 43. https://doi.org/10.3390/ma16010043
Giannakopoulos S, Vakros J, Manariotis ID, Mantzavinos D, Lianos P. Study of the Functionalities of a Biochar Electrode Combined with a Photoelectrochemical Cell. Materials. 2023; 16(1):43. https://doi.org/10.3390/ma16010043
Chicago/Turabian StyleGiannakopoulos, Spyridon, John Vakros, Ioannis D. Manariotis, Dionissios Mantzavinos, and Panagiotis Lianos. 2023. "Study of the Functionalities of a Biochar Electrode Combined with a Photoelectrochemical Cell" Materials 16, no. 1: 43. https://doi.org/10.3390/ma16010043