1. Introduction
With the proposal and gradual development of 5G, communication equipment has higher and higher property requirements for materials. Due to the frequency of communication waves rising to the GHz level, the loss in wave propagation caused by using traditional materials as the substrate of antenna arrays is much greater than before. To solve this problem, it is necessary to find new materials with lower Dk and Df or to optimize the dielectric properties of materials through physical and chemical methods [
1,
2,
3,
4,
5]. PI is widely used in the electronics industry because of its excellent mechanical properties, insulation properties, heat resistance and corrosion resistance [
6,
7]. At present, the material of the receiving antenna group of many smart terminals, such as mobile phones, is PI. The commercial PI materials at present have Dk of more than 3.0 and Df of 10
−2, which is far from meeting the requirements of 5G or even higher frequency communication wave frequencies [
8,
9,
10,
11,
12,
13,
14].
In recent years, many methods have been put forward to improve the dielectric properties of PI. For chemical modification, fluorine-containing or silicon-containing monomers and original dianhydride diamine monomers can be copolymerized to acquire the polymer with the target structure [
15,
16]. This is because of the low polarity of corresponding bonds introduced into the polymer molecular system effectively reducing the molecular polarity and the electronic polarization so as to reduce the Dk. Second, compared with the original monomers, the introduced molecule has a larger molecular weight and more complex molecular structure, increasing the asymmetry of the new molecular structure and the unit mole mass, which reduce the proportion of the easily polarized imide groups in the unit structure, thus reducing the polarity of the molecule and reducing the Dk and Df [
17,
18,
19,
20]. From the aspect of physical modification, the introduction of a hollow structure is very effective. For example, the doping of nanometer hollow microspheres or hollow particles in PAA glue introduces air of low Dk into the polymer system, thus greatly improving the dielectric properties of PIs. Other researchers used light, heat, radiation and initiators to activate monomer molecules into free radicals, which polymerized to produce porous PI materials [
21,
22,
23,
24].
Many teams studying silicon-containing PIs with low permittivity carried out research from the perspective of physical doping, while few cases were studied from the perspective of chemical synthesis, and most of them were commercial patents that were not open to the public.
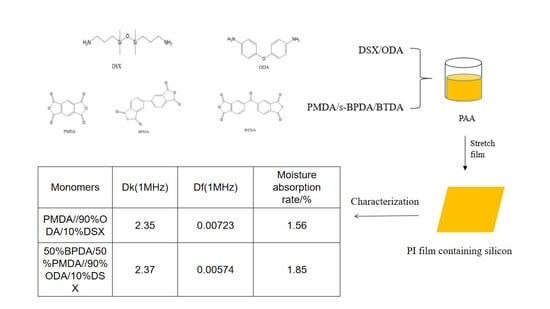
Based on chemical modification, this study focused on the ways to improve the dielectric properties of PIs from the perspective of molecular synthesis. DSX and ODA were mixed in different proportions and randomly polymerized with PMDA, BTDA and BPDA, respectively, and then tetrad-copolymerized with these three dianhydrides to obtain a series of silicon-containing PIs. Meanwhile, the effects of different monomer combinations on the dielectric properties of PI materials were investigated by simulation calculation and synthesis experiments, and the results were generally consistent. The effects of different monomer combinations and frequencies on the dielectric properties of PIs were investigated, and the monomer formulations with relatively good comprehensive properties were selected. It was confirmed that Dk decreased as the silicon content increased, and BPDA was more suitable than BTDA when introduced into the PI structure to decrease Dk and Df. The best formula was when the ratio of diamines was 1:9 and mixed with PMDA. The performance data were as follows: vitrification temperature was about 320 °C, T5 was 551 °C, water absorption was 1.56%, Dk was 2.35, Df was 0.007, tensile strength was 70 MPa and elongation at break was 10.2%.
2. Simulation Calculation
This experiment mainly studied the dielectric properties. According to the relationship between different properties and chemical structure, the Dk and Df were related to the polarizability. Therefore, the polarization was chosen as the target of this simulation calculation, and the molecular results of PI structures polymerized by different monomers were calculated to obtain the magnitude relationship of polarization so as to deduce the changing trend of Dk and its relationship with different monomer structures.
First, we determined that the diamines and dianhydrides involved in the experiment were ODA, DSX, PMDA, s-BPDA and BTDA, respectively, and the PI molecular structures that might be synthesized by ternary or quaternary polymerization of them, as shown in
Figure 1 and
Figure 2. Then, the molecular structures above were imported into the simulation calculation software, Materials Studio (7.0, Accelrys, San Diego, CA, USA), and ‘Polar’ was calculated. Finally, the polarization results were obtained, as shown in
Table 1.
It should be noted that under ideal conditions, all monomers labeled as participating in polymerization were polymerized in the main chain, and only the molecular structure of the main chain covering all kinds of monomers was calculated without considering the difference caused by the number of short chains in polymers and the ratio of monomers. The cross-linking and crystallization during polymerization and imidization were preset (the cross-linking could not be controlled, and crystallization was avoided as far as possible through the experimental heating process and cooling process design).
Since the structures introduced in the simulation were the unit structures of each PI molecule, the volumes of the unit structure containing different amounts of monomers were not equal, so only the structures with the same number of monomers were compared simultaneously. Polarization result 1/Angstrom
3, polarization result 2/cm
3 and polarization result 3/a.u. are three physical quantities representing the polarizability from different views. As can be seen in
Table 1, the introduction of DSX indeed reduced the polarization of the PI molecular structure. However, the data also showed that if ODA was completely replaced with DSX, the polarization would increase somewhat, indicating that when the silicon content reached a certain level, the polarization would decrease adversely. For the polymerization containing DSX, the polarizability of PI films only polymerized with PMDA was lower than that with extra BPDA and BTDA. For the tetrad polymerization containing DSX, the polarizability of the PI film containing PMDA and BPDA was lower than that containing PMDA and BTDA. Therefore, according to the simulation results, the PI films synthesized by ODA/DSX//PMDA and ODA/DSX//BPDA/PMDA had the best dielectric properties in tetrad polymerization.
3. Experiment
3.1. Reagents
PMDA (99%), DSX (97%), ODA (99.5%), N,N-Dimethylacetamide (DMAc), BPDA (97%) and BTDA (99%) were bought from Macklin (Shanghai, China).
3.2. Ternary Polymerization of PMDA, ODA and DSX
In this experiment, the molar ratio of PMDA to diamines was 1:1, and the molar ratios of DSX to ODA were 1:3, 1:5, 1:7, 1:9 to 1:17, respectively, and the solvent was DMAc. After complete dissolution of DMAc and diamine, PMDA was added evenly and slowly for homopolymerization. The experimental conditions were as follows: the temperature was controlled at 24–26 °C, the ambient humidity was about 35%, the PMDA feeding time was controlled within 30–40 min and the stirring speed was constant during feeding. After feeding, the speed was adjusted according to the viscosity of the glue, then continued to react for 6 h, and stirring was stopped. Finally, a series of silicon-containing polyimides were obtained, which was group 1.
3.3. Effects of Different Types of Dianhydride on Properties of Silicon-Containing Polyimides
According to the characterization results of Experiment 3.2, PI films had the best comprehensive performance when the ratio of DSX to ODA was 1:9. Therefore, Experiment 3.3 used the ratio above to explore the influence of different dianhydride combinations on the performance of PI film with DSX structure.
In the ratio of 1:9, ternary homopolymerization with BPDA and BTDA was carried out, respectively. The experimental process was consistent with the above, and silicon-containing polyimide films were obtained by polymerization of different dianhydrides with two kinds of diamines, which was group 2. Then, tetrad polymerizations were carried out with PMDA, BPDA and BTDA, respectively, to obtain a series of silicon-containing polyimides, which was divided into group 3. Films of group 2 and group 3 were successively characterized as above.
3.4. Measurements
The Fourier transform infrared (FTIR) spectra of PI films were obtained using a Nicolet 6700 Fourier Transform Infrared Spectrometer (Thermo scientific, Shanghai, China). Thermogravimetric analysis (TGA) of the polyimides was carried out with a TGA 4000 (PerkinElmer, Waltham, MA, USA) at a heating rate of 10 °C/min from 30 °C to 750 °C under N2 atmosphere. Differential scanning calorimetry (DSC) of the composites was tested by a DSC 8000 (PerkinElmer, Waltham, MA, USA) at a heating rate of 20 °C/min from 30 °C to 450 °C under N2 atmosphere. The dielectric constants and loss were determined by a Dielectric Spectrometer (Wuyi Electronic, Shanghai, China) in the range of 1 Hz to 1 MHz at 29% relative humidity.
The hygroscopicity of PI films was measured according to GB/T1033-1998. The details were as follows.
The PI films were cut into square pieces the size of 5 cm × 5 cm and dried in a 60-degree oven for 24 h. The mass m
1 was then weighed. Then, it was soaked in deionized water for 24 h and weighed immediately after wiping the water on the surface of the film to obtain the mass m
2. Each sample was measured three times and averaged. The hygroscopicity can be obtained by the following formula.
4. Result
4.1. Structural Elucidation: FTIR
As shown in
Figure 3, the bending vibration peak of the C=O bond could be found at 723 cm
−1, the stretching vibration peak of the C-N bond could be seen at 1379 cm
−1 and the stretching vibration peak of the C=O bond could be found at 1776 cm
−1 and 1720 cm
−1, proving that the imide structure was formed in the polymer. The stretching vibration peak of the Si-O-Si bond could be observed at 1054 cm
−1, which proved that the silicon-containing group of DSX was successfully introduced into the molecular structure of PI. The stretching vibration peaks of the C-H bond could be seen at 2927 cm
−1 and 2850 cm
−1, which represented the successful introduction of the aliphatic chain of DSX into PI molecular structures. According to the infrared image, DSX was successfully introduced into the molecular structure of PI without any influence on the synthesis of PI.
Figure 4 shows that the bending vibration peak of the C=O bond could be seen at 723 cm
−1, the stretching vibration peak of the C-N bond could be seen at 1379 cm
−1 and the stretching vibration peaks of the C=O bond were 1776 cm
−1 and 1720 cm
−1, proving that the imide structure was formed in the polymer, which represented the formation of the target PI molecular structure. The stretching vibration peak of the Si-O-Si bond could be observed at 1054 cm
−1, proving that the silicon-containing group of DSX was successfully introduced into the molecular structure of PI.
4.2. Thermal Properties
Since there was no water in PI films, weight loss was directly considered as polymer decomposition. The following TGA curves and T0.5 (the temperature at 0.5% weight loss), T1 (the temperature at 1% weight loss) and T5 (the temperature at 5% weight loss) tables were obtained.
As seen from the curves and
Table 2, the T
5 and the glass transition temperature (Tg) of PI films in group 1 decreased with the increase in DSX in the proportion of diamine. This was because the silicon-containing monomer DSX belonged to aliphatic diamines and had no rigid structure with high-temperature stability, such as a benzene ring. Compared with PI films polymerized by pure ODA and PMDA, however, the Tg and T
5 of PI films in group 1 were all above 300 °C and 500 °C. The thermal stability did not decrease too much and was still within the range required by 5G equipment for the thermal properties of materials. The thermal performance was best when the ratio of DSX to ODA was 1:9, Tg was 318 °C and T
5 was 551 °C.
According to the Tg and T
5 data listed in
Table 3, the T
5 of the BPDA group was above 530 °C, with excellent thermal performance, and the Tg of the BPDA group was 325 °C at the highest and 297 °C at the lowest, which was within the acceptable range. In the BTDA group, although Tg was above 310 °C, the highest T
5 was only 520 °C, showing slightly poor thermal stability compared with the BPDA group. From what has been discussed above, it could be concluded that BPDA was better than BTDA in neutralizing the weakening effect of DSX on the thermal properties of PI films.
4.3. Mechanical Properties
When PI films are applied to 5G, more attention is paid to their flexibility, so the tensile strength and elongation of PI films will be focused on when the mechanical properties are characterized.
As seen in
Table 4, with the decrease in the mole content of DSX in the diamine system, there was no obvious linear relationship between the properties of the polyimide films obtained. Although in the ratio of 1:3 to 1:9, tensile strength and elongation at break increased with the decrease in the DSX proportion, in the whole set of data, tensile strength and elongation at break of 1:9 were not the best. When the ratio was 1:13, PI films had the best mechanical properties, with a tensile strength of 84 MPa and elongation at break of 14.45%.
As seen in
Table 5, when all PMDA was changed into BPDA or BTDA, the tensile strength and elongation at break were decreased, but when only 50% of PMDA was changed into BPDA or BTDA, the tensile strength improved greatly, and the elongation at break did not change much. It could be inferred that the unit molecular structure obtained by polymerization of BPDA and BTDA with DSX was stronger than that of PMDA, resulting in more brittle PI films. However, if only half of PMDA was changed into BPDA or BTDA, due to the uncertain combination of the four monomers, the PI molecule could have a variety of unit structures, and at the same time, it also increased the proportion of benzene ring in the unit molecular structure, so that the tensile strength of polyimide films significantly increased. The formula with the best mechanical properties was 50%BTDA/50%PMDA//90%ODA/10%DSX, with a tensile strength of 142 MPa and elongation at break of 7.12%.
4.4. Dielectric Properties and Hygroscopic
According to the curves shown in
Figure 5, with the increase in frequency, the Dk of the PI film first presented a downward trend and then stabilized within a certain range after decreasing to a certain extent. This was because the chemical structure and composition of DSX are very different from ODA. When DSX and ODA were randomly arranged on both sides of PMDA in aggregation, the molecular structures of the PI were full of uncertainty, and the symmetry was greatly reduced. In addition, the molecular weight of DSX is larger than ODA, making the unit structure of the PI molar volume increase and reducing the proportion of imide groups in the unit structure. Both of the above reasons can effectively reduce the polarization degree of the PI molecule, thus reducing the Dk and Df. As shown in
Figure 5, with the decrease in DSX content in the diamine system, the permittivity of PI films increased and gradually became closer to PI film polymerized by pure PMDA and ODA. It could be observed that the additional amount of DSX had a great influence on the permittivity of PI films. The characterization results showed that the PI films of group 1 all had Df in the range of 10
−3 at 1 MHz, which could meet the requirements of applied industry.
According to the data in
Table 6, in the same size and humidity environment, the water absorption rate of PI films decreased as the ratio of DSX and ODA increased. When the ratio was 1:17, the moisture absorption rate was greater than 2%. This was because the molecular structure of DSX was an aliphatic chain containing a Si-C bond and Si-O-Si bond. Compared with ODA containing a benzene ring and other rigid structures, the intermolecular force of DSX was much smaller. The introduction of DSX into the PI unit structure reduced the viscosity of the new pre-polymer, weakened the surface tension of PI films and reduced the surface energy of PI films. As for hygroscopicity, the hydrophobic ability was enhanced. When the ratio was 1:3, the water absorption of the PI film was the lowest, only 1.18%.
According to the data in
Table 7, DSX could effectively reduce the Dk and Df of PI film in comparison to group 2 or group 3. However, the longitudinal comparison of group 2 and group 3 found that the Dk and Df of ternary quaternion polymerization products of BPDA were both lower than those of BTDA. Therefore, a control group was specially designed for this experiment, namely, ternary polymerization without DSX participation. The Df of BPDA/PMDA//ODA and BTDA/PMDA//ODA was similar to that of BTDA//PMDA/ODA, both of which were in the range of 0.007–0.008. However, the Dk of BPDA was greatly reduced, which meant that BPDA was more suitable for DSX polymerization aiming at enhancing dielectric properties.
As for the moisture absorption rate, the hygroscopies of the formulations in the table above were all higher than 10%DSX/90%ODA//PMDA because in this group of PI films, the favorable structural effect of DSX on reducing hygroscopies was weakened by the decrease in the proportion of DSX to the overall structure. However, the hygroscopicity of PI film obtained by BPDA//90%ODA/10%DSX, 50%BPDA/50%PMDA//90%ODA/10%DSX and 50%BTDA/50%PMDA//90%ODA/10%DSX increased by a relatively small degree. The moisture absorption rate of all PI films was below 2.0%, which meets the requirements of use.
5. Conclusions
In conclusion, BPDA is more suitable than BTDA to introduce a DSX structure diamine polymerization system, which can greatly enhance thermal performance. However, the effect of polymerization of BPDA and a diamine system alone on dielectric properties cannot be ignored, so it is mixed with PMDA in a certain mole ratio. Then, random copolymerization with a diamine system is conducted, which can introduce BPDA’s strong thermal performance structure into the pre-polymerization system while retaining the conventional PMDA structure, playing a role in neutralizing properties. In Experiment 3.2, when the ratio of diamines was 1:9, PI films had the best comprehensive performance. The performance data were as follows: the Vitrification temperature was about 320 °C, T5 was 551 °C, water absorption was 1.56%, Dk was 2.35, Df was 0.007, tensile strength was 70 MPa and elongation at break was 10.2%. In Experiment 3.3, the PI polymerization formula with the best overall performance was DSX:ODA = 1:9, BPDA:PMDA = 1:1, and the properties of the films were as follows: the Dk was 2.37 (1 MHz), Df was 0.00674 (1 MHz), Tg was 325 °C, T5 was 530 °C, moisture absorption was 1.85%, tensile strength was 102 MPa and elongation at break was 9.21%. Compared with the results of the best proportion in Experiment 3.2, the mechanical properties were enhanced, and the excellent dielectric properties, thermal properties and hydrophobic properties were retained.
The above results showed that using the existing dianhydride monomer, DSX and ODA for mixed polymerization could reduce the Dk of PI to 2.1 and Df to 0.0068. Moreover, due to the mature industrial technology of the existing dianhydride diamine monomer, the high purity (even more than 99%) of the monomers could be guaranteed without additional cost, and the preparation process was easy to operate. These advantages make the formula obtained in this study a good fit for large-scale industrial production and could promote the industrialization of the excellent dielectric property PI.
Author Contributions
Conceptualization, Y.C. and Y.L.; methodology, Y.C.; software, Y.C.; validation, Y.C., Y.L. and Y.M.; formal analysis, Y.C.; investigation, Y.C.; resources, Y.C.; data curation, Y.C.; writing—original draft preparation, Y.C.; writing—review and editing, Y.C.; project administration, Y.L.; funding acquisition, Y.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Key R&D Program of China (2020YFB0408100), NSFC (U20A20340), the Program for Guangdong Introducing Innovative and Entrepreneurial Team (2016ZT06C412), Foshan Introducing Innovative and Entrepreneurial Teams (No. 1920001000108) and the Hundred Talent Program of Guangdong University of Technology (220418095).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Acknowledgments
We acknowledge Cui for providing simulating assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- BOC INTERNATIONAL (CHINA). 5G series: Special topics on terminal Antenna. Electron. Secur. Res. Rep. 2019, 2, 25. [Google Scholar]
- Li, L.; Yuan, S.; He, Z. Preparation and Properties of Polyimide/Molecular Sieve. Insul. Mater. 2019, 52, 35–39. [Google Scholar]
- Huang, F.; Meng, G.; Guo, Y.; Zhi, X.-X.; Zhang, Y.; Wu, L.; Jia, Y.-J.; Liu, J.-G. Research and development to low dielectric polymer materials under the background of 5G demand [A]. China Electronic Material Industry Association Copper Clad Material Branch, China Electronic Circuit Industry Association copper clad material branch. In Proceedings of the Copper Clad Panel Material Branch of China Electronic Materials Industry Association, Guangzhou, China, 3–5 December 2020; p. 8. [Google Scholar]
- Yang, Z.; Guo, H.; Gao, L. Structure design and high frequency dielectric properties of polyimide [A]. Copper Clad Material Branch of China Electronic Materials Industry Association, Copper clad Panel Branch of China Electronic Circuit Industry Association. In Proceedings of the 21st China Copper Clad Panel Technology Seminar [C]. Copper Clad Panel Material Branch of China Electronic Materials Industry Association, Copper clad Panel Branch of China Electronic Circuit Industry Association: Copper Clad Panel Material Branch of China Electronic Materials Industry Association, Guangzhou, China, 3–5 December 2020; p. 5. [Google Scholar]
- Ji, Y.; Tang, X.; Liu, Y. Development of Preparation Methods for Low Dielectric Constant Non-Fluorinated Polyimide Film. Insul. Mater. 2016, 49, 28–32. [Google Scholar]
- Li, Z.; Kou, K.; Chen, H. Research Progress in Preparation Methods of Polyimide Materials with Low Dielectric Constant. Eng. Plast. Appl. 2015, 43, 141–144. [Google Scholar]
- Simone, C.D.; Vaccaro, E.; Scola, D.A. The Synthesis and Characterization of Highly Fluorinated Aromatic Polyimides. J. Fluor. Chem. 2019, 224, 100–112. [Google Scholar] [CrossRef]
- Wang, X. Properties of Polyhedral Oligomeric Silsesquioxane/Graphene Oxide/Plyimide Composite Films with Low Dielectric Constant. Ph.D. Thesis, Harbin University of Science and Technology, Harbin, China, 2018. [Google Scholar]
- Qiu, G.; Ma, W.; Wu, L. Low dielectric constant polyimide mixtures fabricated by polyimide matrix and polyimide microsphere fillers. Polym. Int. 2020, 5, 485–491. [Google Scholar] [CrossRef]
- Ge, C.; Ke, L. Preparation and Properties of Low Dielectric Constant Polyimide/Aluminum Acetylacetonate Hybrid Films. China Plast. Ind. 2020, 48, 22–25. [Google Scholar]
- Huang, Z.X. Preparation of Polyimide/Zeolite Hybrid Films with Low Dielectric Constant; South China University of Technology: Guangzhou, China, 2013. [Google Scholar]
- Fan, Z.-G.; Chen, W.-X.; Wei, S.-Y. Quantitative Structure-Property Relationship Study on Dielectric Constant of Polyimide and Its Molecular Structure Design for Low Dielectric Films. Acta Polym. Sin. 2019, 50, 179–188. [Google Scholar]
- Wang, M.; Yang, X.; Yao, H. Preparation of Disiloxane-containing Copolyimide with Low Dielectric Constant and Low Dielectric Loss. Insul. Mater. 2018, 51, 16–21. [Google Scholar]
- He, Z.A. Preparation and Properties of POSS/PI Porous Composite Films with Low Dielectric Properties; Harbin University of Science and Technology: Harbin, China, 2021. [Google Scholar]
- Chen, Z.; Liu, S.; Zhao, J. Poss-norbornene/polyimide composite films with Low dielectric constant and High fracture toughness [A]. Polymer Discipline Committee of Chinese Chemical Society. In Proceedings of the International Symposium on Polymer Science and Technology, Polymer Science Committee of Chinese Chemical Society, Chinese Chemical Society, Chengdu, China, 10–14 October 2017; p. 1. [Google Scholar]
- Wei, D.Y. Synergistic Modification of Low Dielectric Constant Polyimide Composite Films by SiO_2 Hollow Spheres and Go. Ph.D. Thesis, Harbin University of Science and Technology, Harbin, China, 2016. [Google Scholar]
- Zhou, W.Q. Dielectric Properties and Molecular Simulation of Polyimide/Silicon Nitride Films; Harbin University of Science and Technology: Harbin, China, 2020. [Google Scholar]
- Yan, Y.; Xiong, M.; Liu, B.; Ding, Y.; Chen, Z. Low capacitance and highly reliable blind through-silicon-vias(TSVs) with vacuum-assisted spin coating of polyimide dielectric liners. Sci. China Technol. Sci. 2016, 59, 1581–1590. [Google Scholar] [CrossRef]
- Jin, B.; Zhou, L.B.Y.; Wan, X.D.; Liu, M.X.; Zhu, C. Experimental study of laser lift-off of ultra-thin polyimide film for flexible electronics. Sci. China Technol. Sci. 2019, 62, 233–242. [Google Scholar]
- Luo, K.; Song, G.; Wang, Y.; Yu, J.; Zhu, J.; Hu, Z. Low-K and Recyclable High-Performance POSS/Polyimide Composites Based on Diels-Alder Reaction. ACS Appl. Polym. Mater. 2019, 1, 944–952. [Google Scholar] [CrossRef]
- Rajamanickam, R.; Pichaimani, P.; Muthukaruppan, A. Synthesis and studies on phosphazene core-based POSS-reinforced polyimide nanocomposites. Polym. Bull. 2019, 76, 387–407. [Google Scholar]
- Wang, C.-Y.; Chen, W.-T.; Xu, C.; Zhao, X.-Y.; Li, J. Fluorinated Polyimide/POSS Hybrid Polymers with High Solubility and Low Dielectric Constant. Chin. J. Polym. Sci. 2016, 34, 1363–1372. [Google Scholar] [CrossRef]
- Zhou, D.-Y.; Zhang, T.; Wang, X.-D. Synthesis and Properties of Hollow Glass Microspheres/Polyimide Composite Film. China Plast. Ind. 2018, 46, 29–32. [Google Scholar]
- Chen, M.; Zhou, W.; Zhang, J.; Chen, Q. Dielectric Property and Space Charge Behavior of Polyimide/Silicon Nitride Nanocomposite Films. Polymers 2020, 12, 322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).