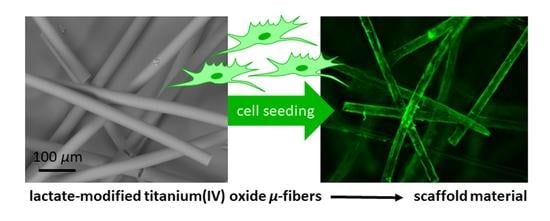
Sol-Gel-Derived Fibers Based on Amorphous α-Hydroxy-Carboxylate-Modified Titanium(IV) Oxide as a 3-Dimensional Scaffold
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sol Synthesis and Fiber Spinning
2.3. Degradation Studies
2.4. Analytical Methods
2.4.1. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray Spectroscopy (EDX)
2.4.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.4.3. X-ray Powder Diffraction (XRD)
2.4.4. 13C Magic Angle Spinning Nuclear Magnetic Resonance Spectroscopy (13C-MAS-NMR)
2.4.5. Thermogravimetric Analysis (TGA)
2.4.6. 1H- and 13C Nuclear Magnetic Resonance Spectroscopy (NMR)
2.5. In Vitro Cell Culture Experiments
2.5.1. WST-1-Assay
2.5.2. Immunofluorescence Stainings and Fluorescence Imaging
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yin, Z.F.; Wu, L.; Yang, H.G.; Su, Y.H. Recent progress in biomedical applications of titanium dioxide. Phys. Chem. Chem. Phys. 2013, 15, 4844–4858. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef] [Green Version]
- Textor, M.; Sittig, C.; Frauchiger, V.; Tosatti, S.; Brunette, D.M. Properties and Biological Significance of Natural Oxide Films on Titanium and Its Alloys. In Titanium in Medicine: Material Science, Surface Science, Engineering, Biological Responses and Medical Applications; Springer: Berlin/Heidelberg, Germany, 2001; pp. 171–230. [Google Scholar]
- Ha, S.W.; Wintermantel, E. Biokompatible Metalle. In Medizintechnik: Life Science Engineering; Wintermantel, E., Ha, S.W., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 191–217. [Google Scholar]
- Zhang, X.; Tiainen, H.; Haugen, H.J. Comparison of titanium dioxide scaffold with commercial bone graft materials through micro-finite element modelling in flow perfusion. Med. Biol. Eng. Comput. 2019, 57, 311–324. [Google Scholar] [CrossRef] [Green Version]
- Köckerling, F.; Schug-Pass, C. What do we know about titanized polypropylene meshes? An evidence-based review of the literature. Hernia 2014, 18, 445–457. [Google Scholar] [CrossRef] [Green Version]
- Baylón, K.; Rodríguez-Camarillo, P.; Elías-Zúñiga, A.; Díaz-Elizondo, J.A.; Gilkerson, R.; Lozano, K. Past, Present and Future of Surgical Meshes: A Review. Membranes 2017, 7, 47. [Google Scholar] [CrossRef]
- Piveteau, L.-D.; Girona, M.I.; Schlapbach, L.; Barboux, P.; Boilot, J.P.; Gasser, B. Thin films of calcium phosphate and titanium dioxide by a sol-gel route: A new method for coating medical implants. J. Mater. Sci. Mater. Med. 1999, 10, 161–167. [Google Scholar] [CrossRef]
- Ullattil, S.G.; Periyat, P. Sol-Gel Synthesis of Titanium Dioxide. In Sol-Gel Materials for Energy, Environment and Electronic Applications; Pillai, S.C., Hehir, S., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 271–283. [Google Scholar]
- Caruso, R.A.; Schattka, J.H.; Greiner, A. Titanium Dioxide Tubes from Sol–Gel Coating of Electrospun Polymer Fibers. Adv. Mater. 2001, 13, 1577–1579. [Google Scholar] [CrossRef]
- Koch, S.; Kessler, M.; Mandel, K.; Dembski, S.; Heuzé, K.; Hackenberg, S. Polycarboxylate ethers: The key towards non-toxic TiO2 nanoparticle stabilisation in physiological solutions. Colloids Surf. B Biointerfaces 2016, 143, 7–14. [Google Scholar] [CrossRef]
- Catauro, M.; Tranquillo, E.; Poggetto, G.D.; Naviglio, S.; Barrino, F. Antibacterial Properties of Sol–Gel Biomaterials with Different Percentages of PEG or PCL. Macromol. Symp. 2020, 389, 1900056. [Google Scholar] [CrossRef]
- Livage, J.; Sanchez, C. Sol-gel chemistry. J. Non-Cryst. Solids 1992, 145, 11–19. [Google Scholar] [CrossRef]
- Salomatina, E.V.; Bityurin, N.M.; Gulenova, M.V.; Gracheva, T.A.; Drozdov, M.N.; Knyazev, A.V.; Kir’yanov, K.V.; Markin, A.V.; Smirnova, L.A. Synthesis, structure, and properties of organic–inorganic nanocomposites containing poly(titanium oxide). J. Mater. Chem. C 2013, 1, 6375–6385. [Google Scholar] [CrossRef]
- Schubert, U. New materials by sol–gel processing: Design at the molecular level. J. Chem. Soc. Dalton Trans. 1996, 16, 3343–3348. [Google Scholar] [CrossRef]
- Schubert, U. Chemical modification of titanium alkoxides for sol–gel processing. J. Mater. Chem. 2005, 15, 3701–3715. [Google Scholar] [CrossRef]
- Gómez-Ruiz, S.; Gallego, B.; Žižak, Ž.; Hey-Hawkins, E.; Juranić, Z.D.; Kaluđerović, G.N. Titanium (IV) carboxylate complexes: Synthesis, structural characterization and cytotoxic activity. Polyhedron 2010, 29, 354–360. [Google Scholar] [CrossRef]
- Kumar, A.; Gaurav; Malik, A.K.; Tewary, D.K.; Singh, B. A review on development of solid phase microextraction fibers by sol–gel methods and their applications. Anal. Chim. Acta 2008, 610, 1–14. [Google Scholar] [CrossRef]
- Kursawe, M.; Glaubitt, W.; Thierauf, A. Biodegradable Silica Fibers from Sols. J. Sol-Gel Sci. Technol. 1998, 13, 267–271. [Google Scholar] [CrossRef]
- Bao, N.; Wei, Z.; Ma, Z.; Liu, F.; Yin, G. Si-doped mesoporous TiO2 continuous fibers: Preparation by centrifugal spinning and photocatalytic properties. J. Hazard. Mater. 2010, 174, 129–136. [Google Scholar] [CrossRef]
- Ghosal, K.; Agatemor, C.; Špitálsky, Z.; Thomas, S.; Kny, E. Electrospinning tissue engineering and wound dressing scaffolds from polymer-titanium dioxide nanocomposites. Chem. Eng. J. 2019, 358, 1262–1278. [Google Scholar] [CrossRef]
- Kim, W.T.; Park, D.C.; Yang, W.H.; Cho, C.H.; Choi, W.Y. Effects of Electrospinning Parameters on the Microstructure of PVP/TiO2 Nanofibers. Nanomaterials 2021, 11, 1616. [Google Scholar] [CrossRef]
- Gupta, K.K.; Kundan, A.; Mishra, P.K.; Srivastava, P.; Mohanty, S.; Singh, N.K.; Mishra, A.; Maiti, P. Polycaprolactone composites with TiO2 for potential nanobiomaterials: Tunable properties using different phases. Phys. Chem. Chem. Phys. 2012, 14, 12844–12853. [Google Scholar] [CrossRef]
- Costa, R.G.F.; Brichi, G.S.; Ribeiro, C.; Mattoso, L.H.C. Nanocomposite fibers of poly (lactic acid)/titanium dioxide prepared by solution blow spinning. Polym. Bull. 2016, 73, 2973–2985. [Google Scholar] [CrossRef]
- Kedem, S.; Rozen, D.; Cohen, Y.; Paz, Y. Enhanced Stability Effect in Composite Polymeric Nanofibers Containing Titanium Dioxide and Carbon Nanotubes. J. Phys. Chem. C 2009, 113, 14893–14899. [Google Scholar] [CrossRef]
- Lee, K.; Lee, S. Multifunctionality of poly (vinyl alcohol) nanofiber webs containing titanium dioxide. J. Appl. Polym. Sci. 2012, 124, 4038–4046. [Google Scholar] [CrossRef]
- Tinoco, A.D.; Incarvito, C.D.; Valentine, A.M. Calorimetric, Spectroscopic, and Model Studies Provide Insight into the Transport of Ti(IV) by Human Serum Transferrin. J. Am. Chem. Soc. 2007, 129, 3444–3454. [Google Scholar] [CrossRef] [PubMed]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospinning of polymeric nanofibers for tissue engineering applications: A review. Tissue Eng. 2006, 12, 1197–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, A.; Bhattarai, N.; Zhang, M. Fabrication and cellular compatibility of aligned chitosan–PCL fibers for nerve tissue regeneration. Carbohydr. Polym. 2011, 85, 149–156. [Google Scholar] [CrossRef]
- Koppes, R.A.; Park, S.; Hood, T.; Jia, X.; Abdolrahim Poorheravi, N.; Achyuta, A.H.; Fink, Y.; Anikeeva, P. Thermally drawn fibers as nerve guidance scaffolds. Biomaterials 2016, 81, 27–35. [Google Scholar] [CrossRef]
- Chen, F.; Wan, H.; Xia, T.; Guo, X.; Wang, H.; Liu, Y.; Li, X. Promoted regeneration of mature blood vessels by electrospun fibers with loaded multiple pDNA-calcium phosphate nanoparticles. Eur. J. Pharm. Biopharm. 2013, 85, 699–710. [Google Scholar] [CrossRef]
- Brown, A.; Augustin, M.; Jünger, M.; Zutt, M.; Dissemond, J.; Rabe, E.; Kaufmann, R.; Simon, M.; Stücker, M.; Karrer, S.; et al. Randomized standard-of-care-controlled trial of a silica gel fibre matrix in the treatment of chronic venous leg ulcers. Eur. J. Dermatol. 2014, 24, 210–216. [Google Scholar] [CrossRef]
- Grotheer, V.; Goergens, M.; Fuchs, P.C.; Dunda, S.; Pallua, N.; Windolf, J.; Suschek, C.V. The performance of an orthosilicic acid-releasing silica gel fiber fleece in wound healing. Biomaterials 2013, 34, 7314–7327. [Google Scholar] [CrossRef]
- Emmert, M.; Witzel, P.; Rothenburger-Glaubitt, M.; Heinrich, D. Nanostructured surfaces of biodegradable silica fibers enhance directed amoeboid cell migration in a microtubule-dependent process. RSC Adv. 2017, 7, 5708–5714. [Google Scholar] [CrossRef] [Green Version]
- Lotz, C.; Schmid, F.F.; Oechsle, E.; Monaghan, M.G.; Walles, H.; Groeber-Becker, F. Cross-linked Collagen Hydrogel Matrix Resisting Contraction to Facilitate Full-Thickness Skin Equivalents. ACS Appl. Mater. Interf. 2017, 9, 20417–20425. [Google Scholar] [CrossRef] [PubMed]
- Deacon, G.B.; Phillips, R.J. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- Camail, M.; Humbert, M.; Margaillan, A.; Riondel, A.; Vernet, J.L. New acrylic titanium polymers: 1. Synthesis and characterisation of new titanium trialkoxide methacrylate monomers prepared via the esterification of methacrylic acid by titanium tetraalkoxides. Polymer 1998, 39, 6525–6531. [Google Scholar] [CrossRef]
- Urlaub, R.; Posset, U.; Thull, R. FT-IR spectroscopic investigations on sol–gel-derived coatings from acid-modified titanium alkoxides. J. Non-Cryst. Solids 2000, 265, 276–284. [Google Scholar] [CrossRef]
- Chen, Y.K.; Lin, Y.F.; Peng, Z.W.; Lin, J.L. Transmission FT-IR Study on the Adsorption and Reactions of Lactic Acid and Poly (lactic acid) on TiO2. J. Phys. Chem. C 2010, 114, 17720–17727. [Google Scholar] [CrossRef]
- Ohya, T.; Ito, M.; Yamada, K.; Ban, T.; Ohya, Y.; Takahashi, Y. Aqueous Titanate Sols from Ti Alkoxide- -Hydroxycarboxylic Acid System and Preparation of Titania Films from the Sols. J. Sol-Gel Sci. Technol. 2004, 30, 71–81. [Google Scholar] [CrossRef]
- Kakihana, M.; Tomita, K.; Petrykin, V.; Tada, M.; Sasaki, S.; Nakamura, Y. Chelating of Titanium by Lactic Acid in the Water-Soluble Diammonium Tris(2-hydroxypropionato)titanate(IV). Inorg. Chem. 2004, 43, 4546–4548. [Google Scholar] [CrossRef]
- Périneau, F.; Pensec, S.; Sassoye, C.; Ribot, F.; van Lokeren, L.; Willem, R.; Bouteiller, L.; Sanchez, C.; Rozes, L. New hybrid core–shell star-like architectures made of poly (n-butyl acrylate) grown from well-defined titanium oxo-clusters. J. Mater. Chem. 2011, 21, 4470–4475. [Google Scholar] [CrossRef]
- Ekoi, E.J.; Stallard, C.; Reid, I.; Dowling, D.P. Tailoring oxide-layer formation on titanium substrates using microwave plasma treatments. Surf. Coat. Technol. 2017, 325, 299–307. [Google Scholar] [CrossRef]
- Seisenbaeva, G.A.; Daniel, G.; Nedelec, J.-M.; Kessler, V.G. Solution equilibrium behind the room-temperature synthesis of nanocrystalline titanium dioxide. Nanoscale 2013, 5, 3330–3336. [Google Scholar] [CrossRef] [PubMed]
- Kakihana, M.; Kobayashi, M.; Tomita, K.; Petrykin, V. Application of Water-Soluble Titanium Complexes as Precursors for Synthesis of Titanium-Containing Oxides via Aqueous Solution Processes. Bull. Chem. Soc. Jpn. 2010, 83, 1285–1308. [Google Scholar] [CrossRef]
- Kobayashi, M.; Osada, M.; Kato, H.; Kakihana, M. Design of crystal structures, morphologies and functionalities of titanium oxide using water-soluble complexes and molecular control agents. Polym. J. 2015, 47, 78–83. [Google Scholar] [CrossRef]
- Patri, A.; Umbreit, T.; Zheng, J.; Nagashima, K.; Goering, P.; Francke-Carroll, S.; Gordon, E.; Weaver, J.; Miller, T.; Sadrieh, N.; et al. Energy dispersive X-ray analysis of titanium dioxide nanoparticle distribution after intravenous and subcutaneous injection in mice. J. Appl. Toxicol. 2009, 29, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Duarte, C.A.; Goulart, L.R.; Filice, L.D.S.C.; de Lima, I.L.; Campos-Fernández, E.; Dantas, N.O.; Silva, A.C.A.; Soares, M.B.P.; dos Santos, R.R.; Cardoso, C.M.A.; et al. Characterization of Crystalline Phase of TiO2 Nanocrystals, Cytotoxicity and Cell Internalization Analysis on Human Adipose Tissue-Derived Mesenchymal Stem Cells. Materials 2020, 13, 4071. [Google Scholar] [CrossRef]
- Wu, W.C.; Chuang, C.C.; Lin, J.L. Bonding Geometry and Reactivity of Methoxy and Ethoxy Groups Adsorbed on Powdered TiO2. J. Phys. Chem. B 2000, 104, 8719–8724. [Google Scholar] [CrossRef]
- Lloyd, S.G.; Zeng, H.; Wang, P.; Chatham, J.C. Lactate isotopomer analysis by 1H NMR spectroscopy: Consideration of long-range nuclear spin–spin interactions. Magn. Reson. Med. 2004, 51, 1279–1282. [Google Scholar] [CrossRef]
- Sugimoto, T.; Zhou, X.; Muramatsu, A. Synthesis of Uniform Anatase TiO2 Nanoparticles by Gel–Sol Method: 1. Solution Chemistry of Ti(OH)n(4−n)+ Complexes. J. Colloid Interface Sci. 2002, 252, 339–346. [Google Scholar] [CrossRef]
- Loza-Rosas, S.A.; Saxena, M.; Delgado, Y.; Gaur, K.; Pandrala, M.; Tinoco, A.D. A ubiquitous metal, difficult to track: Towards an understanding of the regulation of titanium(iv) in humans. Metallomics 2017, 9, 346–356. [Google Scholar] [CrossRef] [Green Version]
- Tsave, O.; Salifoglou, A. Biomimetic activity of soluble, well-defined, aqueous Ti(IV)-citrate species toward adipogenesis. An in vitro study. J. Inorg. Biochem. 2021, 214, 111290. [Google Scholar] [CrossRef]
- Limo, M.J.; Sola-Rabada, A.; Boix, E.; Thota, V.; Westcott, Z.C.; Puddu, V.; Perry, C.C. Interactions between Metal Oxides and Biomolecules: From Fundamental Understanding to Applications. Chem. Rev. 2018, 118, 11118–11193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sibum, H. Titanium and Titanium Alloys—From Raw Material to Semi-finished Products. Adv. Eng. Mater. 2003, 5, 393–398. [Google Scholar] [CrossRef]
- Baramov, T.; Keijzer, K.; Irran, E.; Mösker, E.; Baik, M.-H.; Süssmuth, R. Synthesis and structural characterization of hexacoordinate silicon, germanium, and titanium complexes of the E. coli siderophore enterobactin. Chemistry 2013, 19, 10536–10542. [Google Scholar] [CrossRef] [PubMed]
- Croot, P.L. Rapid Determination of Picomolar Titanium in Seawater with Catalytic Cathodic Stripping Voltammetry. Anal. Chem. 2011, 83, 6395–6400. [Google Scholar] [CrossRef]
- Dumon, J.C.; Ernst, W. Titanium in Plants. J. Plant Physiol. 1988, 133, 203–209. [Google Scholar] [CrossRef]
- Diebold, U. The surface science of titanium dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar] [CrossRef]
- Lu, P.J.; Huang, S.C.; Chen, Y.P.; Chiueh, L.C.; Shih, D.Y.C. Analysis of titanium dioxide and zinc oxide nanoparticles in cosmetics. J. Food Drug Anal. 2015, 23, 587–594. [Google Scholar] [CrossRef] [Green Version]





| n(TEOT) (mol) | n(LA) (mol) | n(H2O) (mol) | n(EtOH) (mol) | |
|---|---|---|---|---|
| 1 | 1.00 | 1.00 | 18.00 | 5.00 |
| 2 | 1.00 | 0.25 | 0.10 | 5.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christ, B.; Glaubitt, W.; Berberich, K.; Weigel, T.; Probst, J.; Sextl, G.; Dembski, S. Sol-Gel-Derived Fibers Based on Amorphous α-Hydroxy-Carboxylate-Modified Titanium(IV) Oxide as a 3-Dimensional Scaffold. Materials 2022, 15, 2752. https://doi.org/10.3390/ma15082752
Christ B, Glaubitt W, Berberich K, Weigel T, Probst J, Sextl G, Dembski S. Sol-Gel-Derived Fibers Based on Amorphous α-Hydroxy-Carboxylate-Modified Titanium(IV) Oxide as a 3-Dimensional Scaffold. Materials. 2022; 15(8):2752. https://doi.org/10.3390/ma15082752
Chicago/Turabian StyleChrist, Bastian, Walther Glaubitt, Katrin Berberich, Tobias Weigel, Jörn Probst, Gerhard Sextl, and Sofia Dembski. 2022. "Sol-Gel-Derived Fibers Based on Amorphous α-Hydroxy-Carboxylate-Modified Titanium(IV) Oxide as a 3-Dimensional Scaffold" Materials 15, no. 8: 2752. https://doi.org/10.3390/ma15082752