Characterization of Hydroxyapatite Film Obtained by Er:YAG Pulsed Laser Deposition on Sandblasted Titanium: An In Vitro Study
Abstract
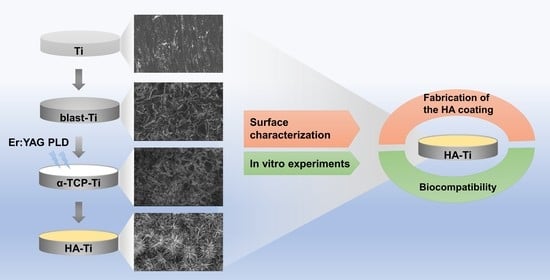
:1. Introduction
2. Materials and Methods
2.1. Fabrication of the Hydroxyapatite Coating
2.2. Characterization
2.3. Cell Cultures
2.4. Attachment and Proliferation of Cells
2.5. Morphology of Cells
2.6. Statistical Analysis
3. Results
3.1. Characterization of the Hydroxyapatite Coating
3.2. Attachment and Proliferation of Cells
3.3. Morphology of Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface Modifications and Their Effects on Titanium Dental Implants. BioMed Res. Int. 2015, 2015, 791725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ottria, L.; Lauritano, D.; Andreasi Bassi, M.; Palmieri, A.; Candotto, V.; Tagliabue, A.; Tettamanti, L. Mechanical, Chemical and Biological Aspects of Titanium and Titanium Alloys in Implant Dentistry. J. Biol. Regul. Homeost. Agents 2018, 32, 81–90. [Google Scholar] [PubMed]
- Xuereb, M.; Camilleri, J.; Attard, N.J. Systematic Review of Current Dental Implant Coating Materials and Novel Coating Techniques. Int. J. Prosthodont. 2015, 28, 51–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puleo, D.A.; Nanci, A. Understanding and Controlling the Bone-Implant Interface. Biomaterials 1999, 20, 2311–2321. [Google Scholar] [CrossRef]
- Bosshardt, D.D.; Chappuis, V.; Buser, D. Osseointegration of Titanium, Titanium Alloy and Zirconia Dental Implants: Current Knowledge and Open Questions. Periodontology 2000 2017, 73, 22–40. [Google Scholar] [CrossRef] [PubMed]
- Kern, M.; Thompson, V.P. Effects of Sandblasting and Silica-Coating Procedures on Pure Titanium. J. Dent. 1994, 22, 300–306. [Google Scholar] [CrossRef]
- Annunziata, M.; Guida, L.; Perillo, L.; Aversa, R.; Passaro, I.; Oliva, A. Biological Response of Human Bone Marrow Stromal Cells to Sandblasted Titanium Nitride-Coated Implant Surfaces. J. Mater. Sci. Mater. Med. 2008, 19, 3585–3591. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Kang, T.; Shin, H.; Kim, K. Osteogenic Properties of Novel Hydroxyapatite Scaffold. Int. J. Mol. Sci. 2020, 21, 8501. [Google Scholar] [CrossRef] [PubMed]
- Guillen-Romero, L.D.; Oropeza-Guzmán, M.T.; López-Maldonado, E.A.; Iglesias, A.L.; Paz-González, J.A.; Ng, T.; Serena-Gómez, E.; Villarreal-Gómez, L.J. Synthetic Hydroxyapatite and Its Use in Bioactive Coatings. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800018817463. [Google Scholar] [CrossRef] [Green Version]
- Uskoković, V.; Uskoković, D.P. Nanosized Hydroxyapatite and Other Calcium Phosphates: Chemistry of Formation and Application as Drug and Gene Delivery Agents. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 96, 152–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, F.; Zhou, H.; Lee, J. Various Preparation Methods of Highly Porous Hydroxyapatite/Polymer Nanoscale Biocomposites for Bone Regeneration. Acta Biomater. 2011, 7, 3813–3828. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Biswas, K.; Basu, B. Hydroxyapatite–Titanium Bulk Composites for Bone Tissue Engineering Applications. J. Biomed. Mater. Res. A 2015, 103, 791–806. [Google Scholar] [CrossRef] [PubMed]
- Kreisler, M.; Kohnen, W.; Marinello, C.; Götz, H.; Duschner, H.; Jansen, B.; d’Hoedt, B. Bactericidal Effect of the Er:YAG Laser on Dental Implant Surfaces: An In Vitro Study. J. Periodontol. 2002, 73, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- AlMoharib, H.S.; Steffensen, B.; Zoukhri, D.; Finkelman, M.; Gyurko, R. Efficacy of an Er:YAG Laser in the Decontamination of Dental Implant Surfaces: An In Vitro Study. J. Periodontol. 2021, 92, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Hympanova, L.; Mackova, K.; El-Domyati, M.; Vodegel, E.; Roovers, J.P.; Bosteels, J.; Krofta, L.; Deprest, J. Effects of Non-Ablative Er:YAG Laser on the Skin and the Vaginal Wall: Systematic Review of the Clinical and Experimental Literature. Int. Urogynecol. J. 2020, 31, 2473–2484. [Google Scholar] [CrossRef] [PubMed]
- Kriechbaumer, L.K.; Happak, W.; Distelmaier, K.; Thalhammer, G.; Kaiser, G.; Kugler, S.; Tan, Y.; Leonhard, M.; Zatorska, B.; Presterl, E.; et al. Disinfection of Contaminated Metal Implants with an Er:YAG Laser. J. Orthop. Res. 2020, 38, 2464–2473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Hontsu, S.; Komasa, S.; Yamamoto, E.; Hashimoto, Y.; Matsumoto, Y. Hydroxyapatite Film Coating by Er:YAG Pulsed Laser Deposition Method for the Repair of Enamel Defects. Materials 2021, 14, 7475. [Google Scholar] [CrossRef]
- Hontsu, S.; Kato, N.; Yamamoto, E.; Yoshikawa, K.; Hashimoto, Y. Fabrication of a HA Films Using the Er: YAG Laser. J. Bio-Integr. Bio Integr. Soc. 2014, 4, 65–69. [Google Scholar]
- Hontsu, S. Preparation of Biomaterial Thin Films by Pulsed-Laser Deposition Technique. Rev. Laser Eng. 2000, 28, 407–412. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Li, Y.L.; Gao, H.Y.; Wang, G.L.; Xia, P. Alpha-Tricalcium Phosphate as a Bone Graft: Research and Development in Orthopaedics. Chin. J. Tissue Eng. Res. 2016, 20, 6494–6500. [Google Scholar] [CrossRef]
- Ma, Y.; Li, S.-W.; Feng, Z.-D. Restoration of Tooth Enamel Caries by Hydrolysis of α-TCP. J. Inorg. Mater. 2009, 24, 275–279. [Google Scholar] [CrossRef]
- Park, Y.M.; Yang, T.Y.; Yoon, S.Y.; Stevens, R.; Park, H.C. Hydrolysis of α-TCP and Development of Hydroxyapatite Whiskers. Key Eng. Mater. 2005, 280, 1511–1514. [Google Scholar] [CrossRef]
- Fernández, E.; Gil, F.J.; Ginebra, M.P.; Driessens, F.C.; Planell, J.A.; Best, S.M. Calcium Phosphate Bone Cement for Clinical Applications. Part I: Solution Chemistry. J. Mater. Sci. Mater. Med. 1999, 10, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.W. Hydration Behavior of Calcium Phosphates is Analogous to Hydration Behavior of Calcium Silicates. Cem. Concr. Res. 1999, 29, 1167–1171. [Google Scholar] [CrossRef]
- Ginebra, M.-P.; Driessens, F.C.M.; Planell, J.A. Effect of the Particle Size on the Micro and Nanostructural Features of a Calcium Phosphate Cement: A Kinetic Analysis. Biomaterials 2004, 25, 3453–3462. [Google Scholar] [CrossRef] [PubMed]
- Ginebra, M.-P.; Fernández, E.; Driessens, F.C.M.; Planell, J.A. Modeling of the Hydrolysis of α-Tricalcium Phosphate. J. Am. Ceram. Soc. 1999, 82, 2808–2812. [Google Scholar] [CrossRef]
- Takeuchi, A.; Munar, M.L.; Wakae, H.; Maruta, M.; Matsuya, S.; Tsuru, K.; Ishikawa, K. Effect of Temperature on Crystallinity of Carbonate Apatite Foam Prepared from α-Tricalcium Phosphate by Hydrothermal Treatment. Biomed. Mater. Eng. 2009, 19, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, M.; Suda, H.; Okamoto, K.; Ioku, K. Hydrothermal Synthesis of Biocompatible Whiskers. J. Mater. Sci. 1994, 29, 3399–3402. [Google Scholar] [CrossRef]
- Durucan, C.; Brown, P.W. Kinetic Model for α–Tricalcium Phosphate Hydrolysis. J. Am. Ceram. Soc. 2002, 85, 2013–2018. [Google Scholar] [CrossRef]
- Ma, M.-G. Hierarchically Nanostructured Hydroxyapatite: Hydrothermal Synthesis, Morphology Control, Growth Mechanism, and Biological Activity. Int. J. Nanomed. 2012, 7, 1781–1791. [Google Scholar] [CrossRef] [Green Version]
- Charbord, P. Bone Marrow Mesenchymal Stem Cells: Historical Overview and Concepts. Hum. Gene Ther. 2010, 21, 1045–1056. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Komasa, S.; Hashimoto, Y.; Hontsu, S.; Okazaki, J. In Vitro and In Vivo Osteogenic Activity of Titanium Implants Coated by Pulsed Laser Deposition with a Thin Film of Fluoridated Hydroxyapatite. Int. J. Mol. Sci. 2018, 19, 1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Komasa, S.; Mashimo, C.; Sekino, T.; Okazaki, J. Effect of Ultraviolet Treatment on Bacterial Attachment and Osteogenic Activity to Alkali-Treated Titanium with Nanonetwork Structures. Int. J. Nanomedicine. 2017, 12, 4633–4646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Li, H.; Wu, J.; Yang, Q.; Jiang, D.; Qiao, B. Polydopamine-induced Hydroxyapatite Coating Facilitates Hydroxyapatite/Polyamide 66 Implant Osteogenesis: An In Vitro and In Vivo Evaluation. Int. J. Nanomedicine. 2018, 13, 8179–8193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Kwun, S.I.; Yoon, J.G. Electrical Properties of Nb-Doped SrTiO3 Thin Films Grown by Pulsed Laser Deposition. J. Korean Phys. Soc. 1998, 32, 214–217. [Google Scholar]
- Gupta, D.; Venugopal, J.; Mitra, S.; Dev, V.R.G.; Ramakrishna, S. Nanostructured Biocomposite Substrates by Electrospinning and Electrospraying for the Mineralization of Osteoblasts. Biomaterials 2009, 30, 2085–2094. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.-H.; Castano, O.; Kim, H.-W. Electrospun Materials as Potential Platforms for Bone Tissue Engineering. Adv. Drug Deliv. Rev. 2009, 61, 1065–1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.; Zhou, J.; Lu, S.; Zhang, L. Enhanced Osteoblast Functions of Narrow Interligand Spaced Sr-HA Nanofibers/rods Grown on Microporous Titania Coatings. RSC Adv. 2013, 3, 11169–11184. [Google Scholar] [CrossRef]
- Han, Y.; Zhou, J.; Zhang, L.; Xu, K. A Multi-Scaled Hybrid Orthopedic Implant: Bone ECM-Shaped Sr-HA Nanofibers on the Microporous Walls of a Macroporous Titanium Scaffold. Nanotechnology 2011, 22, 275603. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.P. Biomimetic Materials for Tissue Engineering. Adv. Drug Deliv. Rev. 2008, 60, 184–198. [Google Scholar] [CrossRef] [Green Version]
- Dalby, M.J.; Gadegaard, N.; Tare, R.; Andar, A.; Riehle, M.O.; Herzyk, P.; Wilkinson, C.D.; Oreffo, R.O. The Control of Human Mesenchymal Cell Differentiation Using Nanoscale Symmetry and Disorder. Nat. Mater. 2007, 6, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.W.; Lin, H.-I.; Ho, J.H.-C.; Shih, Y.R.; Chen, H.F.; Yen, T.J.; Lee, O.K. Regulation of the Fate of Human Mesenchymal Stem Cells by Mechanical and Stereo-Topographical Cues Provided by Silicon Nanowires. Biomaterials 2012, 33, 5013–5022. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Ueda, M.; Kohiga, Y.; Imura, K.; Hontsu, S. Application of fluoridated hydroxyapatite thin film coatings using KrF pulsed laser deposition. Dent. Mater. J. 2018, 37, 408–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukaszewska-Kuska, M.; Wirstlein, P.; Majchrowski, R.; Dorocka-Bobkowska, B. Osteoblastic cell behaviour on modified titanium surfaces. Micron 2018, 105, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Łukaszewska-Kuska, M.; Krawczyk, P.; Martyla, A.; Hędzelek, W.; Dorocka-Bobkowska, B. Hydroxyapatite Coating on Titanium Endosseous Implants for Improved Osseointegration: Physical and Chemical Considerations. Adv. Clin. Exp. Med. 2018, 27, 1055–1059. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Dong, L.L.; He, F.; Zhao, S.; Yang, G.-L. Osteoblast responses to thin nanohydroxyapatite coated on roughened titanium surfaces deposited by an electrochemical process. Oral Sur. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, e311–e316. [Google Scholar] [CrossRef]













Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Li, M.; Komasa, S.; Yan, S.; Yang, Y.; Nishizaki, M.; Chen, L.; Zeng, Y.; Wang, X.; Yamamoto, E.; et al. Characterization of Hydroxyapatite Film Obtained by Er:YAG Pulsed Laser Deposition on Sandblasted Titanium: An In Vitro Study. Materials 2022, 15, 2306. https://doi.org/10.3390/ma15062306
Ma L, Li M, Komasa S, Yan S, Yang Y, Nishizaki M, Chen L, Zeng Y, Wang X, Yamamoto E, et al. Characterization of Hydroxyapatite Film Obtained by Er:YAG Pulsed Laser Deposition on Sandblasted Titanium: An In Vitro Study. Materials. 2022; 15(6):2306. https://doi.org/10.3390/ma15062306
Chicago/Turabian StyleMa, Lin, Min Li, Satoshi Komasa, Sifan Yan, Yuanyuan Yang, Mariko Nishizaki, Liji Chen, Yuhao Zeng, Xin Wang, Ei Yamamoto, and et al. 2022. "Characterization of Hydroxyapatite Film Obtained by Er:YAG Pulsed Laser Deposition on Sandblasted Titanium: An In Vitro Study" Materials 15, no. 6: 2306. https://doi.org/10.3390/ma15062306