Indigo—A New Tribological Substance Class for Non-Toxic and Ecological Gliding Surfaces on Ice, Snow, and Water
Abstract
:1. Introduction
2. Synthesis and Specific Properties of Indigo
2.1. Synthesis of Indigo
2.2. Individual and Collective Properties of Indigo
3. Indigo as a Gliding Surface
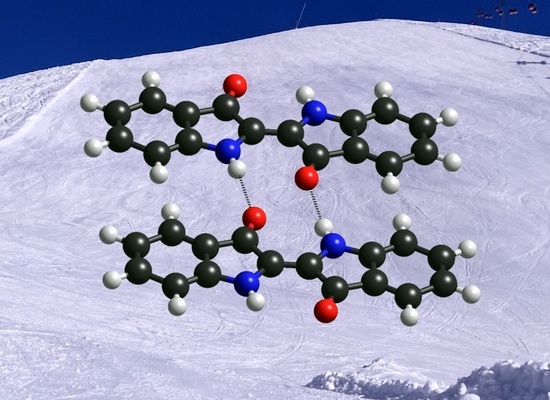
3.1. Self-Assembly of Indigo Molecules
3.2. Gliding Properties of Indigo
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Splitstoser, J.C.; Dillehay, T.D.; Wouters, J.; Claro, A. Early Pre-Hispanic Use of Indigo Blue in Peru. Sci. Adv. 2016, 2, e1501623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doménech, A.; Doménech-Carbó, M.T.; del Río, M.S.; Vázquez de Agredos Pascual, M.L.; Lima, E. Maya Blue as a Nanostructured Polyfunctional Hybrid Organic–Inorganic Material: The Need to Change Paradigms. New J. Chem. 2009, 33, 2371–2379. [Google Scholar] [CrossRef]
- Woodtli, P.; Giger, S.; Müller, P.; Sägesser, L.; Zucchetto, N.; Reber, M.J.; Ecker, A.; Brühwiler, D. Indigo in the Nanochannels of Zeolite L: Towards a New Type of Colorant. Dye. Pigment. 2018, 149, 456–461. [Google Scholar] [CrossRef] [Green Version]
- Hawley, G.G. The Condensed Chemical Dictionary, 9th ed.; Van Nostrand Reinhold Co.: New York, NY, USA, 1977; ISBN 978-0-442-23240-5. [Google Scholar]
- Villagomez, C.J.; Guillermet, O.; Goudeau, S.; Ample, F.; Xu, H.; Coudret, C.; Bouju, X.; Zambelli, T.; Gauthier, S. Self-Assembly of Enantiopure Domains: The Case of Indigo on Cu(111). J. Chem. Phys. 2010, 132, 074705. [Google Scholar] [CrossRef] [PubMed]
- Seligman, T.K.; Loughran, K.; Bernus, E. Art of Being Tuareg: Sahara Nomads in a Modern World; UCLA Fowler Museum of Cultural History: Los Angeles, CA, USA, 2006; ISBN 0-9748729-6-2. [Google Scholar]
- Zhang, L.; Wang, L.; Cunningham, A.B.; Shi, Y.; Wang, Y. Island Blues: Indigenous Knowledge of Indigo-Yielding Plant Species Used by Hainan Miao and Li Dyers on Hainan Island, China. J. Ethnobiol. Ethnomed. 2019, 15, 31. [Google Scholar] [CrossRef] [Green Version]
- Anokhin, D.V.; Leshanskaya, L.I.; Piryazev, A.A.; Susarova, D.K.; Dremova, N.N.; Shcheglov, E.V.; Ivanov, D.A.; Razumov, V.F.; Troshin, P.A. Towards Understanding the Behavior of Indigo Thin Films in Organic Field-Effect Transistors: A Template Effect of the Aliphatic Hydrocarbon Dielectric on the Crystal Structure and Electrical Performance of the Semiconductor. Chem. Commun. 2014, 50, 7639–7641. [Google Scholar] [CrossRef] [Green Version]
- Klimovich, I.V.; Leshanskaya, L.I.; Troyanov, S.I.; Anokhin, D.V.; Novikov, D.V.; Piryazev, A.A.; Ivanov, D.A.; Dremova, N.N.; Troshin, P.A. Design of Indigo Derivatives as Environment-Friendly Organic Semiconductors for Sustainable Organic Electronics. J. Mater. Chem. C 2014, 2, 7621–7631. [Google Scholar] [CrossRef]
- OECD SIDS Indigo Blue, 3H-Indol-3-one, 2-(1,3-Dihydro-3-oxo-2H-indol-2-ylidene)-1,2-dihydro, CAS N°: 482-89-3, SIDS Initial Assessment Report for SIAM 2. 1994. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.006.898 (accessed on 14 December 2021).
- Miranda, M.D. Forensic Analysis of Tattoos and Tattoo Inks; CRC Press: Boca Raton, FL, USA, 2015; ISBN 1-4822-2146-2. [Google Scholar]
- Herrero, M.; Rovira, J.; Nadal, M.; Domingo, J.L. Risk Assessment Due to Dermal Exposure of Trace Elements and Indigo Dye in Jeans: Migration to Artificial Sweat. Environ. Res. 2019, 172, 310–318. [Google Scholar] [CrossRef]
- John, P. Extraction. In Handbook of Natural Colorants; Bechtold, T., Mussak, R., Eds.; Wiley: Hoboken, NJ, USA, 2009; ISBN 0-470-51199-0. [Google Scholar]
- Chia, M.A.; Musa, R.I. Effect of Indigo Dye Effluent on the Growth, Biomass Production and Phenotypic Plasticity of Scenedesmus Quadricauda (Chlorococcales). An. Acad. Bras. Cienc. 2014, 86, 419–428. [Google Scholar] [CrossRef] [Green Version]
- Bützer, M.; Bützer, P. Gleitmittel für Wintersportgeräte auf Basis Indigoider Moleküle. Patent WO2019145282A1, 1 August 2019. Available online: https://patents.google.com/patent/WO2019145282A1/de (accessed on 10 January 2022).
- Scheffer, J. The History of Lapland. Available online: https://www.gutenberg.org/ebooks/59695 (accessed on 6 January 2022).
- Gambaretto, G.P. Solid Lubricant and Process for Preparing It. Patent EP0132879A2, 3 February 1985. Available online: https://patents.google.com/patent/EP0132879A2/en (accessed on 10 January 2022).
- ECHA: Scientific Committees Support Further Restrictions of PFAS. Available online: https://echa.europa.eu/-/scientific-committees-support-further-restrictions-of-pfas (accessed on 6 January 2022).
- Brennan, N.M.; Evans, A.T.; Fritz, M.K.; Peak, S.A.; von Holst, H.E. Trends in the Regulation of Per- and Polyfluoroalkyl Substances (PFAS): A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 10900. [Google Scholar] [CrossRef]
- Update on FIS Fluorinated Ski Wax Ban. Available online: https://www.fis-ski.com/en/international-ski-federation/news-multimedia/news/update-on-fis-fluorinated-ski-wax-ban (accessed on 6 January 2022).
- Saling, P.; Kicherer, A.; Dittrich-Krämer, B.; Wittlinger, R.; Zombik, W.; Schmidt, I.; Schrott, W.; Schmidt, S. Eco-Efficiency Analysis by BASF: The Method. Int. J. Life Cycle Assess. 2002, 7, 203–218. [Google Scholar] [CrossRef]
- Plitzko, I.; Mohn, T.; Sedlacek, N.; Hamburger, M. Composition of Indigo Naturalis. Planta Med. 2009, 75, 860–863. [Google Scholar] [CrossRef] [PubMed]
- Fabara, A.; Fraaije, M. An Overview of Microbial Indigo-Forming Enzymes. Appl. Microbiol. Biotechnol. 2020, 104, 925–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aerts, O.; Duchateau, N.; Lambert, J.; Bechtold, T. Sodium Metabisulfite in Blue Jeans: An Unexpected Cause of Textile Contact Dermatitis. Contact Dermat. 2014, 70, 190–192. [Google Scholar] [CrossRef] [PubMed]
- Bektaş, İ.; Karaman, Ş.; Dıraz, E.; Çelik, M. The Role of Natural Indigo Dye in Alleviation of Genotoxicity of Sodium Dithionite as a Reducing Agent. Cytotechnology 2016, 68, 2245–2255. [Google Scholar] [CrossRef]
- Cordin, M.; Bechtold, T.; Pham, T. Quantification of Aniline and N-Methylaniline in Indigo. Sci. Rep. 2021, 11, 21135. [Google Scholar] [CrossRef]
- Fritsche, J. Ueber Das Anilin, Ein Neues Zersetzungsproduct Des Indigo. J. Prakt. Chem. 1840, 20, 453–459. [Google Scholar] [CrossRef] [Green Version]
- Rannug, U.; Bramstedt, H.; Nilsson, U. The Presence of Genotoxic and Bioactive Components in Indigo Dyed Fabrics—A Possible Health Risk? Mutat. Res. 1992, 282, 219–225. [Google Scholar] [CrossRef]
- PubChem CID 10215. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/10215 (accessed on 20 October 2021).
- ChemIDplus. Available online: https://chem.nlm.nih.gov/chemidplus/rn/482-89-3 (accessed on 23 November 2021).
- Vetter, J. Toxins of Amanita Phalloides. Toxicon 1998, 36, 13–24. [Google Scholar] [CrossRef]
- Chan, T.Y.K. Aconite Poisoning. Clin. Toxicol. 2009, 47, 279–285. [Google Scholar] [CrossRef]
- ECHA. 2-(1,3-Dihydro-3-oxo-2H-indol-2-ylidene)-1,2-dihydro-3H-indol-3-one. Available online: https://echa.europa.eu/registration-dossier/-/registered-dossier/14413/4/3 (accessed on 23 November 2021).
- Ferber, K.H. Toxicology of Indigo. A Review. J. Environ. Pathol. Toxicol. Oncol. 1987, 7, 73–83. [Google Scholar] [PubMed]
- Williams, A.J.; Grulke, C.M.; Edwards, J.; McEachran, A.D.; Mansouri, K.; Baker, N.C.; Patlewicz, G.; Shah, I.; Wambaugh, J.F.; Judson, R.S.; et al. The CompTox Chemistry Dashboard: A Community Data Resource for Environmental Chemistry. J. Cheminform. 2017, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Bützer, P. QSAR Data of Indigo, Indirubin, and Isoindigotin, Including Tautomers [Data Set]. Zenodo 2021. [Google Scholar] [CrossRef]
- Vazirisereshk, M.R.; Martini, A.; Strubbe, D.A.; Baykara, M.Z. Solid Lubrication with MoS2: A Review. Lubricants 2019, 7, 57. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Cho, D.-H.; Park, B.H.; Choi, J.S. Nanotribology of 2D Materials and Their Macroscopic Applications. J. Phys. Appl. Phys. 2020, 53, 393001. [Google Scholar] [CrossRef]
- Luan, B.; Ruhong, Z. Wettability and Friction of Water on a MoS2 Nanosheet. Appl. Phys. Lett. 2016, 108, 131601. [Google Scholar] [CrossRef]
- Süsse, P.; Steins, M.; Kupcik, V. Indigo: Crystal structure refinement based on synchrotron data. Z. Krist. Cryst. Mater. 1988, 184, 269–274. [Google Scholar] [CrossRef]
- Klessinger, M.; Lüttke, W. Theoretische und spektroskopische Untersuchungen an Indigofarbstoffen, III. Der Einfluß zwischenmolekularer Wasserstoffbrücken auf die Spektren von Indigo im festen Zustand. Chem. Ber. 1966, 99, 2136–2145. [Google Scholar] [CrossRef]
- Raffay, L.J. Abhandlung über Indigo: Inaugural-Dissertation; Typis Leopoldi Grund; Österreichische Nationalbibliothek: Vienna, Austria, 1835. [Google Scholar]
- Głowacki, E.D.; Voss, G.; Leonat, L.; Irimia-Vladu, M.; Bauer, S.; Sariciftci, N.S. Indigo and Tyrian Purple—From Ancient Natural Dyes to Modern Organic Semiconductors. Isr. J. Chem. 2012, 52, 540–551. [Google Scholar] [CrossRef]
- Beilby, G.T.; Neville, F.H. Surface Flow in Crystalline Solids under Mechanical Disturbance. Proc. R. Soc. Lond. 1904, 72, 218–225. [Google Scholar] [CrossRef] [Green Version]
- Beilby, G.T.; Neville, F.H. The Effect of Heat and of Solvents on Thin Films of Metal. Proc. R. Soc. Lond. 1904, 72, 226–235. [Google Scholar] [CrossRef]
- Cadalbert, M. Analyse der Zusammensetzung und der Morphologie von Indigo-Partikeln im Hochleistungsgleitmittel Isantin; Zürich University of Applied Sciences: Wädenswil, Switzerland, 2021. [Google Scholar]
- PubChem CID 5354391. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5354391 (accessed on 20 October 2021).
- Vautier, M.; Guillard, C.; Herrmann, J.-M. Photocatalytic Degradation of Dyes in Water: Case Study of Indigo and of Indigo Carmine. J. Catal. 2001, 201, 46–59. [Google Scholar] [CrossRef]
- GESTIS-Stoffdatenbank. Available online: https://gestis.dguv.de/data?name=491987 (accessed on 20 October 2021).
- Japan National Institute of Technology and Evaluation (NITE). Chemical Risk Information Platform (CHRIP), CAS-Nr. 482-89-3. Available online: http://www.safe.nite.go.jp/japan/db.html (accessed on 23 November 2021).
- Toxicity Estimation Software Tool (T.E.S.T.) v5.1.1; U.S. Environmental Protection Agency (EPA): Washington, DC, USA, 2021.
- Exposure Assessment Tools and Models. Estimation Program Interface (EPI) v4.1.1; U.S. Environmental Protection Agency (EPA): Washington, DC, USA, 2019.
- National Toxicology Program (NTP), OPERA: Open Structure Activity Relationship App; U.S. Department of Health and Human Services: Washington, DC, USA, 2021.
- Virtual Models for Property Evaluation of Chemicals within a Global Architecture (VEGA); Istituto di Ricerche Farmacologiche Mario Negri, Istituto di Ricovero e Cura a Carattere Ccientifico (IRCCS): Milan, Italy, 2020.
- Gospodinova, N.; Tomsik, E. Hydrogen-Bonding versus π-π Stacking in the Design of Organic Semiconductors: From Dyes to Oligomers. Prog. Polym. Sci. 2014, 43, 33–47. [Google Scholar] [CrossRef]
- Zhao, X.-J.; Hou, H.; Fan, X.-T.; Wang, Y.; Liu, Y.-M.; Tang, C.; Liu, S.-H.; Ding, P.-P.; Cheng, J.; Lin, D.-H.; et al. Molecular Bilayer Graphene. Nat. Commun. 2019, 10, 3057. [Google Scholar] [CrossRef]
- Głowacki, E.D.; Voss, G.; Demirak, K.; Havlicek, M.; Sünger, N.; Okur, A.C.; Monkowius, U.; Gąsiorowski, J.; Leonat, L.; Sariciftci, N.S. A Facile Protection–Deprotection Route for Obtaining Indigo Pigments as Thin Films and Their Applications in Organic Bulk Heterojunctions. Chem. Commun. 2013, 49, 6063–6065. [Google Scholar] [CrossRef]
- Mayrhofer, L.; Moras, G.; Mulakaluri, N.; Rajagopalan, S.; Stevens, P.A.; Moseler, M. Fluorine-Terminated Diamond Surfaces as Dense Dipole Lattices: The Electrostatic Origin of Polar Hydrophobicity. J. Am. Chem. Soc. 2016, 138, 4018–4028. [Google Scholar] [CrossRef]
- Dellamatrice, P.M.; Silva-Stenico, M.E.; de Moraes, L.A.B.; Fiore, M.F.; Monteiro, R.T.R. Degradation of Textile Dyes by Cyanobacteria. Braz. J. Microbiol. 2017, 48, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Faraday, M.I. Note on Regelation. Proc. R. Soc. Lond. 1860, 10, 440–450. [Google Scholar] [CrossRef]
- McConnel, J.C.; Glazebrook, R.T. VI. On the Plasticity of an Ice Crystal. (Preliminary Note). Proc. R. Soc. Lond. 1891, 48, 259–260. [Google Scholar] [CrossRef]
- Bowden, F.P.; Hughes, T.P.; Desch, C.H. The Mechanism of Sliding on Ice and Snow. Proc. R. Soc. Lond. Ser. Math. Phys. Sci. 1939, 172, 280–298. [Google Scholar] [CrossRef]
- Michaelides, A.; Slater, B. Melting the Ice One Layer at a Time. Proc. Natl. Acad. Sci. USA 2017, 114, 195–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canale, L.; Comtet, J.; Niguès, A.; Cohen, C.; Clanet, C.; Siria, A.; Bocquet, L. Nanorheology of Interfacial Water during Ice Gliding. Phys. Rev. X 2019, 9, 041025. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Qian, L. Role of Interfacial Water in Adhesion, Friction, and Wear—A Critical Review. Friction 2021, 9, 1–28. [Google Scholar] [CrossRef]
- Swarén, M.; Karlöf, L.; Holmberg, H.-C.; Eriksson, A. Validation of Test Setup to Evaluate Glide Performance in Skis. Sports Technol. 2014, 7, 89–97. [Google Scholar] [CrossRef]
- Breitschädel, F. A New Approach for the Grinding of Nordic Skis. Procedia Eng. 2015, 112, 385–390. [Google Scholar] [CrossRef] [Green Version]
- Al-Godari, N. Charakterisierung Indigoider Gleitschichten auf UHMWPE; Zürich University of Applied Sciences: Wädenswil, Switzerland, 2020. [Google Scholar]
- Rhyner, H.; Zeilinger, F.; Duelli, A. Isantin B3 als Gleitmittel für Ski-Beläge; Technical Report; WSL Institute for Snow and Avalanche Research SLF: Davos, Switzerland, 2019. [Google Scholar]
- Bruhin, B.; Neff, A. Internal Test Report on Isantin; Swiss-Ski: Muri bei Bern, Switzerland, 2021. [Google Scholar]






 |  |  | |
|---|---|---|---|
| water solubility (mg/L, 25 °C) | exp.: 0.05–2 mg/L pred.: 7.19 a, 13.44 b, 1.0 c | pred.: 59.92 a, 6.36 b, 1.8 c | pred.: 35.68 a, 0.411 b, 8.2 c |
| log(Kow) | exp.: 2.7–3.72 pred.: 3.11 a,1.99 ± 0.63 d | pred.: 4.10 a, 2.31 ± 1.50 d | pred.: 5.49 a, 2.53 ± 2.58 d |
| log(BCF) | exp.: 2.5–4.5 pred.: 5.6 a, 1.5 b, 0.8 ± 0.2 c | pred.: 4.1 a, 1.8 b, 1.6 ± 1.0 c | pred.: 3.8 a, 3.3 b, 2.7 ± 1.7 c |
| pKa | pred.: 9.00 c | pred.: 8.16 c | pred.: 5.17 c |
| Snow Temperature | ||||
|---|---|---|---|---|
| Cold <−8 °C | Medium −8 °C to −1 °C | Warm >−1 °C | ||
| reference ski (HF) | ski with HF | 0.008 (4) | −0.003 (2) | −0.033 (5) |
| ski with indigo | 0.022 (4) | −0.005 (2) | 0.024 (5) | |
| reference ski (NF) | ski with HF | (0) | −0.008 (1) | −0.089 (3) |
| ski with indigo | (0) | 0.024 (1) | 0.057 (3) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bützer, P.; Brühwiler, D.; Bützer, M.R.; Al-Godari, N.; Cadalbert, M.; Giger, M.; Schär, S. Indigo—A New Tribological Substance Class for Non-Toxic and Ecological Gliding Surfaces on Ice, Snow, and Water. Materials 2022, 15, 883. https://doi.org/10.3390/ma15030883
Bützer P, Brühwiler D, Bützer MR, Al-Godari N, Cadalbert M, Giger M, Schär S. Indigo—A New Tribological Substance Class for Non-Toxic and Ecological Gliding Surfaces on Ice, Snow, and Water. Materials. 2022; 15(3):883. https://doi.org/10.3390/ma15030883
Chicago/Turabian StyleBützer, Peter, Dominik Brühwiler, Marcel Roland Bützer, Nassim Al-Godari, Michelle Cadalbert, Mathias Giger, and Sandro Schär. 2022. "Indigo—A New Tribological Substance Class for Non-Toxic and Ecological Gliding Surfaces on Ice, Snow, and Water" Materials 15, no. 3: 883. https://doi.org/10.3390/ma15030883