Facile Synthesis of CoOOH Nanorings over Reduced Graphene Oxide and Their Application in the Reduction of p-Nitrophenol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
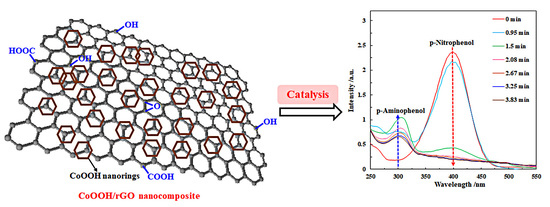
2.2. Synthesis of CoOOH/rGO Nanocomposite
2.3. Catalyst Characterization
2.4. Catalytic Reduction of p-NP to p-AP by NaBH4
3. Results and Discussion
3.1. Characterization of Catalyst
3.2. Catalytic Reduction of p-Nitrophenol
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dragos-Pinzaru, O.G.; Buema, G.; Gherca, D.; Tabakovic, I.; Lupu, N. Effect of the preparation conditions on the catalytic properties of copt for highly efficient 4-nitrophenol reduction. Materials 2022, 15, 6250. [Google Scholar] [CrossRef] [PubMed]
- Dan, H.; Song, Y.; Xu, Y.; Gao, Y.; Kong, W.; Huang, Y.; Yue, Q.; Gao, B. Green synthesis of Cu nanoparticles supported on straw-graphene composite for catalytic reduction of p-nitrophenol. J. Clean. Prod. 2021, 283, 124578. [Google Scholar] [CrossRef]
- Akbarzadeh, E.; Bahrami, F.; Gholami, M.R. Au and Pt nanoparticles supported on Ni promoted MoS2 as efficient catalysts for p-nitrophenol reduction. J. Water Process. Eng. 2020, 34, 101142. [Google Scholar] [CrossRef]
- Geng, L.; Li, G.; Zhang, X.; Wang, X.; Li, C.; Liu, Z.; Zhang, D.S.; Zhang, Y.Z.; Wang, G.; Han, H. Rational design of CuO/SiO2 nanocatalyst with anchor structure and hydrophilic surface for efficient hydrogenation of nitrophenol. J. Solid State. Chem. 2021, 296, 121960. [Google Scholar] [CrossRef]
- Liao, G.; Gong, Y.; Zhong, L.; Fang, J.; Zhang, L.; Xu, Z.; Gao, H.; Fang, B. Unlocking the door to highly efficient Ag-based nanoparticles catalysts for NaBH4-assisted nitrophenol reduction. Nano Res. 2019, 12, 2407–2436. [Google Scholar] [CrossRef]
- Din, M.I.; Khalid, R.; Hussain, Z.; Hussain, T.; Mujahid, A.; Najeeb, J.; Izhar, F. Nanocatalytic assemblies for catalytic reduction of nitrophenols: A critical review. Crit. Rev. Anal. Chem. 2020, 50, 322–338. [Google Scholar] [CrossRef]
- Kotp, M.G.; El-Mahdy, A.F.; Yang, T.L.; Kuo, S.W. A pyridinyl-phenanzine conjugated microporous polymer decorated with ultrafine Ag nanoparticles mediates the rapid reduction of nitrophenol. Micropor. Mesopor. Mat. 2022, 331, 111669. [Google Scholar] [CrossRef]
- Mohamed, H.O.; Lotfi, B.T.; Abdel, H.H. Synthesis of ultra-small gold nanoparticles by polyphenol extracted from Salvia officinalis and efficiency for catalytic reduction of p-nitrophenol and methylene blue. Green Chem. Lett. Rev. 2020, 13, 18–26. [Google Scholar]
- Ayman, M.M.; Eman, A.M.; Nasser, S.A.; Hala, A.I. Catalytic activity of Ag nanoparticles and Au/Ag nanocomposite prepared by pulsed laser ablation technique against 4-nitrophenol for environmental applications. J. Mater. Sci. Mater. Electron. 2021, 32, 11978–11988. [Google Scholar]
- Swarnalata, S.; Bhavya, M.B.; Vishal, K.; Prangya, B.; Akshaya, K.S.; Siddappa, A.P. Controlled synthesis of palladium nanocubes as an efficient nanocatalyst for Suzuki−Miyaura cross-coupling and reduction of p-nitrophenol. Langmuir 2020, 36, 5208–5218. [Google Scholar]
- Ji-Hyang, N.; Reinout, M. Synthesis and catalytic evaluation of dendrimer-templated and reverse microemulsion Pd and Pt nanoparticles in the reduction of 4-nitrophenol: The effect of size and synthetic methodologies. Appl. Catal. A Gen. 2015, 497, 107–120. [Google Scholar]
- Hasan, Z.; Ok, Y.S.; Rinklebe, J.; Tsang, Y.F.; Cho, D.W.; Song, H. N doped cobalt-carbon composite for reduction of p-nitrophenol and pendimethaline. J. Alloy. Compd. 2017, 703, 118–124. [Google Scholar] [CrossRef]
- Somasundaram, S.; Ill-Min, C.; Vanaraj, R.; Ramaganthan, B.; Mayakrishnan, G. Highly active and reducing agent-free preparation of cost-effective NiO-based carbon nanocomposite and its application in reduction reactions under mild conditions. J. Ind. Eng. Chem. 2018, 60, 91–101. [Google Scholar] [CrossRef]
- Che, W.; Ni, Y.; Zhang, Y.; Ma, Y. Morphology-controllable synthesis of CuO nanostructures and their catalytic activity for the reduction of 4-nitrophenol. J. Phys. Chem. Solids. 2015, 77, 1–7. [Google Scholar] [CrossRef]
- Mogudi, B.M.; Ncube, P.; Meijboom, R. Catalytic activity of mesoporous cobalt oxides with controlled porosity and crystallite sizes: Evaluation using the reduction of 4-nitrophenol. Appl. Catal. B Environ. 2016, 198, 74–82. [Google Scholar] [CrossRef]
- Elfiad, A.; Galli, F.; Djadoun, A.; Sennour, M.; Chegrouche, S.; Meddour-Boukhobza, L.; Boffito, D.C. Natural α-Fe2O3 as an efficient catalyst for the p-nitrophenol reduction. Mater. Sci. Eng. B 2018, 229, 126–134. [Google Scholar] [CrossRef]
- Zhang, S.; Zhong, L.; Xu, Z.; Hu, J.; Tang, A.; Zuo, X. Mineral-modulated Co catalyst with enhanced adsorption and dissociation of BH4− for hydrogenation of p-nitrophenol to p-aminophenol. Chemosphere 2022, 291, 132871. [Google Scholar] [CrossRef]
- Hasan, Z.; Cho, D.W.; Chon, C.M.; Yoon, K.; Song, H. Reduction of p-nitrophenol by magnetic Co-carbon composites derived from metal organic frameworks. Chem. Eng. J. 2016, 298, 183–190. [Google Scholar] [CrossRef]
- Shi, X.; Quan, S.; Yang, L.; Shi, G.; Shi, F. Facile synthesis of magnetic Co3O4/BFO nanocomposite for effective reduction of nitrophenol isomers. Chemosphere 2019, 219, 914–922. [Google Scholar] [CrossRef]
- Chen, H.; Yang, M.; Tao, S.; Chen, G. Oxygen vacancy enhanced catalytic activity of reduced Co3O4 towards p-nitrophenol reduction. Appl. Catal. B Environ. 2017, 209, 648–656. [Google Scholar] [CrossRef]
- Upadhyay, D.; Roondhe, B.; Pratap, A.; Jha, P.K. Two-dimensional delafossite cobalt oxyhydroxide as a toxic gas sensor. Appl. Surf. Sci. 2019, 476, 198–204. [Google Scholar] [CrossRef]
- Dhawale, D.S.; Kim, S.; Park, D.H.; Choy, J.H.; Al-deyab, S.S.; Ariga, K.; Kim, E.; Vinu, A. Hierarchically ordered porous CoOOH thin film electrodes for high performance supercapacitors. ChemElectroChem 2015, 2, 497–502. [Google Scholar] [CrossRef] [Green Version]
- Seyyedin, S.T.; Yaftian, M.R.; Sovizi, M.R. Cobalt oxyhydroxide/graphene oxide nanocomposite for amelioration of electrochemical performance of lithium/sulfur batteries. J. Solid State Electrochem. 2017, 21, 649–656. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, T.; Wen, H.; Ni, Z.; He, Y.; Guo, R.; You, J.; Liu, X. The latest development of CoOOH two-dimensional materials used as OER catalysts. Chem. Comm. 2020, 56, 15387–15405. [Google Scholar] [CrossRef]
- Ren, Q.; Feng, Z.; Mo, S.; Huang, C.; Li, S.; Zhang, W.; Chen, L.; Fu, M.; Wu, J.; Ye, D. 1D-Co3O4, 2D-Co3O4, 3D-Co3O4 for catalytic oxidation of toluene. Catal. Today 2019, 332, 160–167. [Google Scholar] [CrossRef]
- Hong, Q.L.; Zhai, Q.G.; Liang, X.L.; Yang, Y.; Li, F.M.; Jiang, Y.C.; Hu, M.C.; Li, S.N.; Chen, Y. Holey cobalt oxyhydroxide nanosheets for the oxygen evolution reaction. J. Mater. Chem. A 2021, 9, 3297–3302. [Google Scholar] [CrossRef]
- Su, Y.; Zhu, Y.; Jiang, H.; Shen, J.; Yang, X.; Zou, W.; Chen, J.; Li, C. Cobalt nanoparticles embedded in N-doped carbon as an efficient bifunctional electrocatalyst for oxygen reduction and evolution reactions. Nanoscale 2014, 6, 15080–15089. [Google Scholar] [CrossRef]
- Yang, Q.; Choi, H.; Chen, Y.; Dionysiou, D.D. Heterogeneous activation of peroxymonosulfate by supported cobalt catalysts for the degradation of 2, 4-dichlorophenol in water: The effect of support, cobalt precursor, and UV radiation. Appl. Catal. B Environ. 2008, 77, 300–307. [Google Scholar] [CrossRef]
- Ndolomingo, M.J.; Bingwa, N.; Meijboom, R. Review of supported metal nanoparticles: Synthesis methodologies, advantages and application as catalysts. J. Mater. Sci. 2020, 55, 6195–6241. [Google Scholar] [CrossRef]
- Zhang, M.; Su, X.; Ma, L.; Khan, A.; Wang, L.; Wang, J.; Maloletnev, A.; Yang, C. Promotion effects of halloysite nanotubes on catalytic activity of Co3O4 nanoparticles toward reduction of 4-nitrophenol and organic dyes. J. Hazard. Mater. 2021, 403, 123870. [Google Scholar] [CrossRef]
- Żółtowska, S.; Miñambres, J.F.; Piasecki, A.; Mertens, F.; Jesionowski, T. Three-dimensional commercial-sponge-derived Co3O4@ C catalysts for effective treatments of organic contaminants. J. Environ. Chem. Eng. 2021, 9, 105631. [Google Scholar] [CrossRef]
- Hareesh, K.; Joshi, R.P.; Sunitha, D.V.; Bhoraskar, V.N.; Dhole, S.D. Anchoring of Ag-Au alloy nanoparticles on reduced graphene oxide sheets for the reduction of 4-nitrophenol. Appl. Surf. Sci. 2016, 389, 1050–1055. [Google Scholar]
- An, X.; Li, K.; Tang, J. Cu2O/reduced graphene oxide composites for the photocatalytic conversion of CO2. ChemSusChem 2014, 7, 1086–1093. [Google Scholar] [CrossRef] [Green Version]
- Kang, X.; Teng, D.; Wu, S.; Tian, Z.; Liu, J.; Li, P.; Ma, Y.; Liang, C. Ultrafine copper nanoparticles anchored on reduced graphene oxide present excellent catalytic performance toward 4-nitrophenol reduction. J. Colloid Interf. Sci. 2020, 566, 265–270. [Google Scholar] [CrossRef]
- Zhao, Q.; Bu, D.; Li, Z.; Zhang, X.; Di, L. Cold plasma preparation of Pd/Graphene catalyst for reduction of p-nitrophenol. Nanomaterials 2021, 11, 1341. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, L.; Liu, Y.; Duan, Q. Preparation of reduced-graphene-oxide-supported CoPt and Ag nanoparticles for the catalytic reduction of 4-nitrophenol. Catalysts 2021, 11, 1336. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Y.; Wang, H.; Zhou, J.; Wang, J.; Regier, T.; Dai, H. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 2011, 10, 780–786. [Google Scholar] [CrossRef] [Green Version]
- Dong, C.; Wu, K.L.; Wei, X.W.; Li, X.Z.; Liu, L.; Ding, T.H.; Wang, J.; Ye, Y. Synthesis of graphene oxide-Ag2CO3 composites with improved photoactivity and anti-photocorrosion. CrystEngComm 2014, 16, 730–736. [Google Scholar] [CrossRef]
- Tang, F.; Cheng, W.; Huang, Y.; Su, H.; Yao, T.; Liu, Q.; Liu, J.; Hu, F.; Jiang, Y.; Sun, Z. Strong surface hydrophilicity in Co-based electrocatalysts for water oxidation. ACS Appl. Mater. Interfaces 2017, 9, 26867–26873. [Google Scholar] [CrossRef]
- Jacob, B.; Mohan, M.; Dhanyaprabha, K.; Thomas, H. NiCo2O4 nanoparticles anchored on reduced graphene oxide with enhanced catalytic activity towards the reduction of p-nitrophenol in water. Colloids Surf. A Physicochem. Eng. Asp. 2022, 643, 128717. [Google Scholar] [CrossRef]
- Zhu, L.; Wu, W.; Zhu, Y.; Tang, W.; Wu, Y. Composite of CoOOH nanoplates with multiwalled carbon nanotubes as superior cathode material for supercapacitors. J. Phys. Chem. C 2015, 119, 7069–7075. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Entezari, M.H. Sono-synthesis approach in uniform loading of ultrafine Ag nanoparticles on reduced graphene oxide nanosheets: An efficient catalyst for the reduction of 4-nitrophenol. Ultrason. Sonochem 2018, 44, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Quiñonez, J.L.; Pal, U. Borohydride-assisted surface activation of Co3O4/CoFe2O4 composite and its catalytic activity for 4-nitrophenol reduction. ACS Omega 2019, 4, 10129–10139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chernykh, M.; Mikheeva, N.; Zaikovskii, V.; Salaev, M.; Liotta, L.F.; Mamontov, G. Room-temperature nitrophenol reduction over Ag-CeO2 catalysts: The role of catalyst preparation method. Catalysts 2020, 10, 580. [Google Scholar] [CrossRef]
- Hu, L.; Liu, X.; Guo, A.; Wu, J.; Wang, Y.; Long, Y.; Fan, G. Cobalt with porous carbon architecture: Towards of 4-nitrophenol degradation and reduction. Sep. Purif. Technol. 2022, 288, 120595. [Google Scholar] [CrossRef]
- Zuo, F.; Bozhilov, K.; Dillon, R.J.; Wang, L.; Smith, P.; Zhao, X.; Bardeen, C.; Feng, P. Active facets on titanium (III)-doped TiO2: An effective strategy to improve the visible-light photocatalytic activity. Angew. Chem. Int. Ed. 2012, 51, 6223–6226. [Google Scholar] [CrossRef]
- Zhang, S.; Chang, C.R.; Huang, Z.Q.; Li, J.; Wu, Z.; Ma, Y.; Zhang, Z.; Wang, Y.; Qu, Y. High catalytic activity and chemoselectivity of sub-nanometric Pd clusters on porous nanorods of CeO2 for hydrogenation of nitroarenes. J. Am. Chem. Soc. 2016, 138, 2629–2637. [Google Scholar] [CrossRef]
- Al Nafiey, A.; Addad, A.; Sieber, B.; Chastanet, G.; Barras, A.; Szunerits, S.; Boukherroub, R. Reduced graphene oxide decorated with Co3O4 nanoparticles (rGO-Co3O4) nanocomposite: A reusable catalyst for highly efficient reduction of 4-nitrophenol, and Cr (VI) and dye removal from aqueous solutions. Chem. Eng. J. 2017, 322, 375–384. [Google Scholar] [CrossRef]
- Fang, J.; Chen, X.; Wu, Y.; Liu, H. Facile and green synthesis of Au nanorods/graphene oxide nanocomposite with excellent catalytic properties for reduction of 4-nitrophenol. J. Mater. Sci. 2020, 55, 5880–5891. [Google Scholar] [CrossRef]
- Li, S.; Guo, S.; Yang, H.; Gou, G.; Ren, R.; Li, J.; Dong, Z.; Jin, J.; Ma, J. Enhancing catalytic performance of Au catalysts by noncovalent functionalized graphene using functional ionic liquids. J. Hazard. Mater. 2014, 270, 11–17. [Google Scholar]
- Tian, Y.; Liu, Y.; Pang, F.; Wang, F.; Zhang, X. Green synthesis of nanostructed Ni-reduced graphene oxide hybrids and their application for catalytic reduction of 4-nitrophenol. Colloids Surf. A 2015, 464, 96–103. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, C.; Huang, X.; Li, L.; Yu, N.; Xie, H.; Zhu, Z.; Yuan, Y.; Zhou, L. Two-dimensional MXene enabled carbon quantum dots@ Ag with enhanced catalytic activity towards the reduction of p-nitrophenol. RSC Adv. 2022, 12, 4836–4842. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.S.; Zhu, X.Y.; Meng, H.B.; Feng, J.J.; Wang, A.J. One-pot synthesis of highly branched Pt@Ag core-shell nanoparticles as a recyclable catalyst with dramatically boosting the catalytic performance for 4-nitrophenol reduction. J. Colloid Interf. Sci. 2019, 538, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Li, J.; Song, S.; Li, L.; Lin, J.; Zhang, B.; Li, J. The synthesis of 3D graphene/Au composites via γ-ray irradiation and their use for catalytic reduction of 4-nitrophenol. Nanotechnology 2020, 31, 235604. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Su, Y.; Cheng, M.; Liu, J.; Hou, S. Gold and cobalt nanoparticles dispersed on N-doped carbon matrix as a catalyst for 4-nitrophenol reduction. ChemistrySelect 2022, 7, e202103739. [Google Scholar] [CrossRef]
- Yu, B.; Han, B.; Jiang, X.; Zhou, C.; Xia, K.; Gao, Q.; Wu, J. Toward high activity and durability: An oxygen-rich boron nitride-supported Au nanoparticles for 4-nitrophenol hydrogenation. J. Phys.Chem. C 2019, 123, 10389–10397. [Google Scholar] [CrossRef]
- Tu, W.; Cheng, J.; Yang, R.; Guo, Z.; Yu, D.; Sheng, Z.; Zhao, J.; Song, H.; Song, Y.; Yang, F. The Co3O4 nanosheet hybridized with silver nanoparticles affords long-acting synergetic antimicrobial and catalytic degradation activity. J. Alloy. Compd. 2022, 914, 165284. [Google Scholar] [CrossRef]
- Landge, V.K.; Sonawane, S.H.; Manickam, S.; Babu, G.U.B.; Boczkaj, G. Ultrasound-assisted wet-impregnation of Ag-Co nanoparticles on cellulose nanofibers: Enhanced catalytic hydrogenation of 4-nitrophenol. J. Environ. Chem. Eng. 2021, 9, 105719. [Google Scholar] [CrossRef]
- Kong, C.; Lei, W.; Lei, B.; Pu, F.; Wang, G.; Zhang, X.; Zhou, C.; Yang, Z. CoFe nanoparticle-decorated reduced graphene oxide for the highly efficient reduction of 4-nitrophenol. Langmuir 2021, 37, 10987–10993. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, N.; Geng, L.; Fu, J.; Hu, H.; Zhang, D.; Zhu, B.; Carozza, J.; Han, H. Facile synthesis of ultrafine cobalt oxides embedded into N-doped carbon with superior activity in hydrogenation of 4-nitrophenol. J. Colloid Interf. Sci. 2018, 512, 844–852. [Google Scholar] [CrossRef]
- Gulati, A.; Malik, J.; Kakkar, R. Peanut shell biotemplate to fabricate porous magnetic Co3O4 coral reef and its catalytic properties for p-nitrophenol reduction and oxidative dye degradation. Colloids Surf. A 2020, 604, 125328. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Meng, R.; Jian, P.; Wang, L. Silver nanoparticles-decorated-Co3O4 porous sheets as efficient catalysts for the liquid-phase hydrogenation reduction of p-nitrophenol. J. Colloid Interface Sci. 2019, 551, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, X.; Qin, L.; Mu, J.; Kang, S.Z. Co3O4/CoP composite hollow polyhedron: A superior catalyst with dramatic efficiency and stability for the room temperature reduction of 4-nitrophenol. Appl. Surf. Sci. 2018, 434, 967–974. [Google Scholar] [CrossRef]
- Kuroda, K.; Ishida, T.; Haruta, M. Reduction of 4-nitrophenol to 4-aminophenol over Au nanoparticles deposited on PMMA. J. Mol. Catal. A Chem. 2009, 298, 7–11. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Zhu, Z.; Li, L.; Guo, X.; Lincoln, S.F.; Prud’homme, R.K. Cooperative catalytic activity of cyclodextrin and Ag nanoparticles immobilized on spherical polyelectrolyte brushes. AIChE J. 2014, 60, 1977–1982. [Google Scholar] [CrossRef]










| Samples | BET Surface Area (m2/g) | Total Pore Volume (cc/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| pure CoOOH | 72.6 | 0.29 | 3.5 |
| CoOOH/rGO | 68.2 | 0.34 | 3.4 |
| Catalyst | t0/min |
|---|---|
| CoOOH/rGO | t0 < 0.95 |
| pure CoOOH | 5.67 < t0 < 6.33 |
| Catalyst | Cp-NP | CNaBH4 | wcat | kapp | knor a | Reference |
|---|---|---|---|---|---|---|
| mM | mM | g/L | min−1 | L·min−1·g−1 | ||
| Au (small) | 0.19 | 1.37 | 0.003 | 0.15 | 44.82 | [8] |
| Au (large) | 0.19 | 1.37 | 0.003 | 0.03 | 7.28 | [8] |
| Pt@Ag | 0.09 | 0.01 | 0.016 | 0.36 | 21.66 | [53] |
| Pd | 0.07 | 1.65 | 0.046 | 0.73 | 15.80 | [10] |
| Graphene/Au | 1.41 | 139.53 | 0.465 | 2.00 | 4.30 | [54] |
| Au/Co@N-C | 0.23 | 23.43 | 0.031 | 2.25 | 71.92 | [55] |
| Au/BNO | 1.61 | 96.77 | 0.323 | 2.25 | 6.98 | [56] |
| AuNi/MoS2 | 0.10 | 4.76 | 0.318 | 1.06 | 3.34 | [3] |
| rGO-Ag | 0.20 | 52.87 | 0.080 | 1.18 | 14.75 | [42] |
| Ag/Co3O4 | 0.54 | 53.57 | 0.054 | 2.88 | 53.76 | [57] |
| Ag-Co/CNFs | 0.09 | 18.18 | 0.303 | 1.03 | 3.41 | [58] |
| Pd/graphene | 0.28 | 6.98 | 0.093 | 0.67 | 7.16 | [35] |
| CoFe/rGO | 0.09 | 10.00 | 0.667 | 4.61 | 6.92 | [59] |
| Co/PCNS | 0.09 | 40.98 | 0.033 | 0.31 | 9.60 | [45] |
| Co/CoO/Co3O4-NC | 0.14 | 0.67 | 0.202 | 0.66 | 3.29 | [8] |
| NiCo2O4/rGO | 0.58 | 16.67 | 0.083 | 0.71 | 8.56 | [40] |
| CoOx/CN | 0.32 | 40.00 | 0.080 | 0.25 | 3.15 | [60] |
| Co3O4/HNTs | 0.12 | 46.40 | 0.033 | 0.26 | 7.96 | [30] |
| Co3O4@C | 8.33 | 8.33 | 1.667 | 0.76 | 0.45 | [31] |
| Co3O4 | 0.04 | 15.86 | 0.100 | 0.14 | 1.41 | [61] |
| CoOOH/rGO | 0.13 | 12.50 | 0.071 | 1.77 | 24.78 | This work |
| CoOOH | 0.13 | 12.50 | 0.071 | 1.22 | 17.08 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Yang, M.; Yue, J.; Chen, G. Facile Synthesis of CoOOH Nanorings over Reduced Graphene Oxide and Their Application in the Reduction of p-Nitrophenol. Materials 2022, 15, 8862. https://doi.org/10.3390/ma15248862
Chen H, Yang M, Yue J, Chen G. Facile Synthesis of CoOOH Nanorings over Reduced Graphene Oxide and Their Application in the Reduction of p-Nitrophenol. Materials. 2022; 15(24):8862. https://doi.org/10.3390/ma15248862
Chicago/Turabian StyleChen, Huihui, Mei Yang, Jun Yue, and Guangwen Chen. 2022. "Facile Synthesis of CoOOH Nanorings over Reduced Graphene Oxide and Their Application in the Reduction of p-Nitrophenol" Materials 15, no. 24: 8862. https://doi.org/10.3390/ma15248862