Dielectric and Spin-Glass Magnetic Properties of the A-Site Columnar-Ordered Quadruple Perovskite Sm2CuMn(MnTi3)O12
Abstract
:1. Introduction
2. Experimental
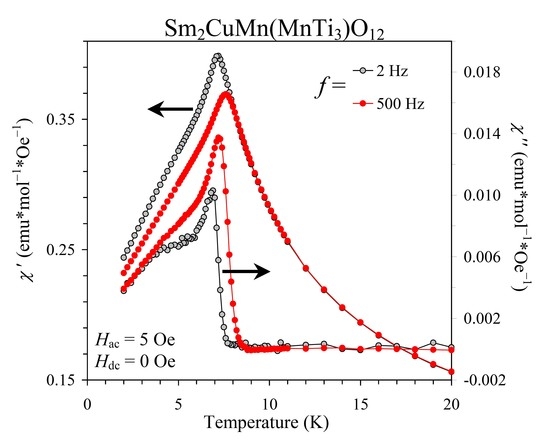
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abakumov, A.M.; Tsirlin, A.A.; Antipov, E.V. Transition-Metal Perovskites. In Comprehensive Inorganic Chemistry II (Second Edition): From Elements to Applications; Reedijk, J., Poeppelmeier, K.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 2, pp. 1–40. [Google Scholar]
- King, G.; Woodward, P.M. Cation ordering in perovskites. J. Mater. Chem. 2010, 20, 5785–5796. [Google Scholar] [CrossRef]
- Vasala, S.; Karppinen, M. A2B′B′′O6 perovskites: A review. Prog. Solid State Chem. 2015, 43, 1–36. [Google Scholar] [CrossRef]
- Knapp, M.C.; Woodward, P.M. A-site cation ordering in AA′BB′O6 perovskites. J. Solid State Chem. 2006, 179, 1076–1085. [Google Scholar] [CrossRef]
- Rubel, M.H.K.; Miura, A.; Takei, T.; Kumada, N.; Mozahar Ali, M.; Nagao, M.; Watauchi, S.; Tanaka, I.; Oka, K.; Azuma, M.; et al. Superconducting double perovskite bismuth oxide prepared by a low-temperature hydrothermal reaction. Angew. Chem. Int. Ed. 2014, 126, 3673–3677. [Google Scholar]
- Rubel, M.H.K.; Takei, T.; Kumada, N.; Mozahar Ali, M.; Miura, A.; Tadanaga, K.; Oka, K.; Azuma, M.; Yashima, M.; Fujii, K.; et al. Hydrothermal synthesis, crystal structure, and superconductivity of a double-perovskite Bi oxide. Chem. Mater. 2016, 28, 459–465. [Google Scholar]
- Vasil’ev, A.N.; Volkova, O.S. New functional materials AC3B4O12 (review). Low Temp. Phys. 2007, 33, 895–914. [Google Scholar] [CrossRef]
- Long, Y.W. A-site ordered quadruple perovskite oxides AA′3B4O12. Chin. Phys. B 2016, 25, 078108. [Google Scholar] [CrossRef]
- Belik, A.A.; Johnson, R.D.; Khalyavin, D.D. The rich physics of A-site-ordered quadruple perovskite manganites AMn7O12. Dalton Trans. 2021, 50, 15458–15472. [Google Scholar] [CrossRef]
- Belik, A.A. Rise of A-site columnar-ordered A2A′A″B4O12 quadruple perovskites with intrinsic triple order. Dalton Trans. 2018, 47, 3209–3217. [Google Scholar] [CrossRef] [Green Version]
- Vibhakar, A.M.; Khalyavin, D.D.; Manuel, P.; Liu, J.; Belik, A.A.; Johnson, R.D. Spontaneous rotation of ferrimagnetism driven by antiferromagnetic spin canting. Phys. Rev. Lett. 2020, 124, 127201. [Google Scholar]
- Zhang, L.; Matsushita, Y.; Yamaura, K.; Belik, A.A. Five-fold ordering in high-pressure perovskites RMn3O6 (R = Gd-Tm and Y). Inorg. Chem. 2017, 56, 5210–5218. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.D.; Khalyavin, D.D.; Manuel, P.; Zhang, L.; Yamaura, K.; Belik, A.A. Magnetic structures of the rare-earth quadruple perovskite manganites RMn7O12. Phys. Rev. B Condens. Matter Mater. Phys. 2018, 98, 104423. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Benitez, J.; Alonso, J.A.; Martinez-Lope, M.J.; de Andres, A.; Fernandez-Diaz, M.T. Enhancement of the Curie temperature along the perovskite series RCu3Mn4O12 driven by chemical pressure of R3+ cations (R = rare earths). Inorg. Chem. 2010, 49, 5679–5685. [Google Scholar] [CrossRef] [PubMed]
- Belik, A.A.; Khalyavin, D.D.; Zhang, L.; Matsushita, Y.; Katsuya, Y.; Tanaka, M.; Johnson, R.D.; Yamaura, K. Intrinsic triple order in A-site columnar-ordered quadruple perovskites: Proof of concept. ChemPhysChem 2018, 19, 2449–2452. [Google Scholar] [CrossRef]
- Belik, A.A.; Matsushita, Y.; Khalyavin, D.D. Reentrant structural transitions and collapse of charge and orbital orders in quadruple perovskites. Angew. Chem. Int. Ed. 2017, 56, 10423–10427. [Google Scholar] [CrossRef]
- Khalyavin, D.D.; Johnson, R.D.; Orlandi, F.; Radaelli, P.G.; Manuel, P.; Belik, A.A. Emergent helical texture of electric dipoles. Science 2020, 369, 680–684. [Google Scholar] [CrossRef]
- Belik, A.A.; Matsushita, Y.; Tanaka, M.; Johnson, R.D.; Khalyavin, D.D. A plethora of structural transitions, distortions and modulations in Cu-doped BiMn7O12 quadruple perovskites. J. Mater. Chem. C 2021, 9, 10232–10242. [Google Scholar] [CrossRef]
- Kayser, P.; Martinez-Lope, M.J.; Alonso, J.A.; Sanchez-Benitez, J.; Fernandez, M.T. High-pressure synthesis and characterization of BiCu3(Mn4−xFex)O12 (x = 0, 1.0, 2.0) complex perovskites. J. Solid State Chem. 2013, 204, 78–85. [Google Scholar] [CrossRef]
- Liu, R.; Khalyavin, D.D.; Tsunoda, N.; Kumagai, Y.; Oba, F.; Katsuya, Y.; Tanaka, M.; Yamaura, K.; Belik, A.A. Spin-glass magnetic properties of A-site columnar-ordered quadruple perovskites Y2MnGa(Mn4–xGax)O12 with 0 ≤ x ≤ 3. Inorg. Chem. 2019, 58, 14830–14841. [Google Scholar]
- Belik, A.A.; Khalyavin, D.D.; Matsushita, Y.; Yamaura, K. Triple A-site cation ordering in the ferrimagnetic Y2CuGaMn4O12 perovskite. Inorg. Chem. 2022, 61, 14428–14435. [Google Scholar] [CrossRef]
- Belik, A.A.; Zhang, L.; Liu, R.; Khalyavin, D.D.; Katsuya, Y.; Tanaka, M.; Yamaura, K. Valence variations by B-site doping in A-site columnar-ordered quadruple perovskites Sm2MnMn(Mn4−xTix)O12 with 1 ≤ x ≤ 3. Inorg. Chem. 2019, 58, 3492–3501. [Google Scholar] [CrossRef]
- Vibhakar, A.M.; Khalyavin, D.D.; Manuel, P.; Liu, R.; Yamaura, K.; Belik, A.A.; Johnson, R.D. Effects of magnetic dilution in the ferrimagnetic columnar ordered Sm2MnMnMn4−xTixO12 perovskites. Phys. Rev. B Condens. Matter Mater. Phys. 2020, 102, 214428. [Google Scholar] [CrossRef]
- Aimi, A.; Mori, D.; Hiraki, K.; Takahashi, T.; Shan, Y.J.; Shirako, Y.; Zhou, J.S.; Inaguma, Y. High-pressure synthesis of A-site ordered double perovskite CaMnTi2O6 and ferroelectricity driven by coupling of A-site ordering and the second-order Jahn–Teller effect. Chem. Mater. 2014, 26, 2601–2608. [Google Scholar] [CrossRef]
- Liu, R.; Scatena, R.; Khalyavin, D.D.; Johnson, R.D.; Inaguma, Y.; Tanaka, M.; Matsushita, Y.; Yamaura, K.; Belik, A.A. High-pressure synthesis, crystal structures, and properties of A-site columnar-ordered quadruple perovskites NaRMn2Ti4O12 with R = Sm, Eu, Gd, Dy, Ho, Y. Inorg. Chem. 2020, 59, 9065–9076. [Google Scholar] [CrossRef]
- Tanaka, M.; Katsuya, Y.; Matsushita, Y.; Sakata, O. Development of a synchrotron powder diffractometer with a one-dimensional X-ray detector for analysis of advanced materials. J. Ceram. Soc. Jpn. 2013, 121, 287–290. [Google Scholar] [CrossRef] [Green Version]
- Izumi, F.; Ikeda, T. A Rietveld-analysis program RIETAN-98 and its applications to zeolites. Mater. Sci. Forum 2000, 321, 198–205. [Google Scholar] [CrossRef]
- Waroquiers, D.; Gonze, X.; Rignanese, G.-M.; Welker-Nieuwoudt, C.; Rosowski, F.; Gobel, M.; Schenk, S.; Degelmann, P.; André, R.; Glaum, R.; et al. Statistical analysis of coordination environments in oxides. Chem. Mater. 2017, 29, 8346–8360. [Google Scholar] [CrossRef]
- Liu, R.; Tanaka, M.; Mori, H.; Inaguma, Y.; Yamaura, K.; Belik, A.A. Ferrimagnetic and relaxor ferroelectric properties of R2MnMn(MnTi3)O12 perovskites with R = Nd, Eu, and Gd. J. Mater. Chem. C 2021, 9, 947–956. [Google Scholar] [CrossRef]
- Mydosh, J.A. Spin Glass: An Experimental Introduction; Taylor & Francis: London, UK, 1993. [Google Scholar]
- von Lohneysen, H. Low energy excitations in amorphous metals. Phys. Rep. 1981, 79, 161–212. [Google Scholar] [CrossRef]
- Binder, K.; Young, A.P. Spin-glasses: Experimental facts, theoretical concepts, and open questions. Rev. Mod. Phys. 1986, 58, 801–976. [Google Scholar] [CrossRef]
- Kittel, C. Introduction to Solid State Physics, 8th ed.; John Wiley and Sons, Inc.: New York, NY, USA, 2005. [Google Scholar]
- Belik, A.A.; Liu, R.; Yamaura, K. Spin-glass to long-range order to spin-glass evolution of magnetic properties with the composition in Sm2MnZnMn4−xTixO12 (x = 1, 2, and 3) perovskites. J. Alloys Compd. 2023, 932, 166297. [Google Scholar] [CrossRef]










| Crystal system | Tetragonal |
| Space group | P42/nmc (No. 137, cell choice 2) |
| Z | 2 |
| Caclulated density (g/cm3) | 6.15 |
| Formula weight (g/cm3) | 809.737 |
| Used d range (Å) | 0.6507–6.238 |
| a (Å) | 7.53477(1) |
| c (Å) | 7.69788(1) |
| V (Å3) | 437.0301(8) |
| g(Sm) | 0.9413(9)Sm + 0.0587Mn |
| z(Sm) | 0.22194(4) |
| B(Sm) (Å2) | 0.796(7) |
| g(Cu) | 0.4597MC + 0.0403Sm |
| z(Cu) | 0.7804(3) |
| B(Cu) (Å2) | 1.25(7) |
| g(Mn) | 0.963(3)Mn + 0.037Sm |
| B(Mn) (Å2) | 0.38(6) |
| g(Ti) | 0.25MC + 0.75Ti |
| B(Ti) (Å2) | 0.400(9) |
| y(O1) | 0.0571(3) |
| z(O1) | −0.0379(3) |
| B(O1) (Å2) | 0.33(5) |
| y(O2) | 0.5363(3) |
| z(O2) | 0.5745(3) |
| B(O2) (Å2) | 0.36(5) |
| x(O3) | 0.44161(23) |
| B(O3) (Å2) | 1.48(7) |
| Rwp (%) | 3.16 |
| Rp (%) | 2.17 |
| RI (%) | 2.63 |
| RF (%) | 1.69 |
| Sm–O1 × 2 | 2.352(3) |
| Sm–O1 × 2 | 2.472(2) |
| Sm–O2 × 2 | 2.437(2) |
| Sm–O3 × 4 | 2.744(1) |
| Cu–O3 × 4 | 2.055(2) |
| Mn–O2 × 4 | 2.102(3) |
| Ti–O1 × 2 | 1.954(1) |
| Ti–O2 × 2 | 1.988(1) |
| Ti–O3 × 2 | 2.023(1) |
| Δ(TiO6) | 2.0 × 10−4 |
| Ti–O1–Ti × 2 | 149.17(9) |
| Ti–O2–Ti × 2 | 142.75(9) |
| Ti–O3–Ti × 2 | 144.17(9) |
| T (K) | M0 | MSG | tr (s) | β |
|---|---|---|---|---|
| 2 | 22.80(13) | 23.67(18) | 1270(24) | 0.4362(4) |
| 3 | 26.01(12) | 27.20(20) | 979(16) | 0.4461(4) |
| 4 | 22.81(10) | 23.94(18) | 800(11) | 0.4495(4) |
| 5 | 17.74(6) | 18.62(13) | 671(8) | 0.4558(4) |
| 6 | 12.34(14) | 13.01(9) | 554(6) | 0.4654(4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belik, A.A.; Liu, R.; Yamaura, K. Dielectric and Spin-Glass Magnetic Properties of the A-Site Columnar-Ordered Quadruple Perovskite Sm2CuMn(MnTi3)O12. Materials 2022, 15, 8306. https://doi.org/10.3390/ma15238306
Belik AA, Liu R, Yamaura K. Dielectric and Spin-Glass Magnetic Properties of the A-Site Columnar-Ordered Quadruple Perovskite Sm2CuMn(MnTi3)O12. Materials. 2022; 15(23):8306. https://doi.org/10.3390/ma15238306
Chicago/Turabian StyleBelik, Alexei A., Ran Liu, and Kazunari Yamaura. 2022. "Dielectric and Spin-Glass Magnetic Properties of the A-Site Columnar-Ordered Quadruple Perovskite Sm2CuMn(MnTi3)O12" Materials 15, no. 23: 8306. https://doi.org/10.3390/ma15238306