Silver Nanostructured Substrates in LDI-MS of Low Molecular Weight Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
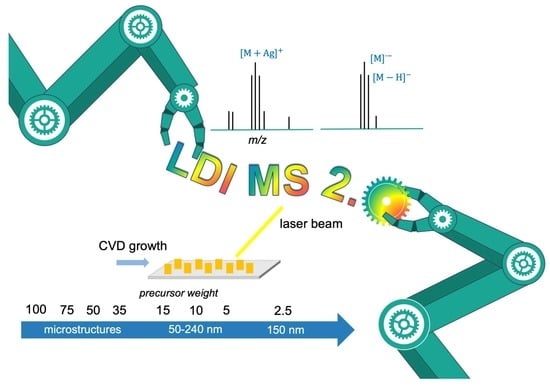
2.2. Synthesis and Characterization of LDI-MS Plates
2.3. LDI-MS Analysis
3. Results and Discussion
3.1. Characterization of LDI Plates
3.2. LDI-MS Performances of Silver Nanostructures for Low Molecular Weight Biomolecules
3.3. LDI-MS Performances of Silver Nanostructures for Lipids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Su, H.; Li, X.; Huang, L.; Cao, J.; Zhang, M.; Vedarethinam, V.; Di, W.; Hu, Z.; Qian, K. Plasmonic Alloys Reveal a Distinct Metabolic Phenotype of Early Gastric Cancer. Adv. Mater. 2021, 33, 2007978. [Google Scholar] [CrossRef] [PubMed]
- Ossoliński, K.; Nizioł, J.; Arendowski, A.; Ossolińska, A.; Ossoliński, T.; Kucharz, J.; Wiechno, P.; Ruman, T. Mass Spectrometry-Based Metabolomic Profiling of Prostate Cancer—A Pilot Study. J. Cancer Metastasis Treat. 2019, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Marin, V.R.; Moreno, M.C.; Villasenor, M.C.G.; Hernandez, E.A.G.; Mendoza, A.G. Presence of Aflatoxin Carcinogens in Fresh and Mature Cheeses. Pharm. Anal. Acta 2018, 9, 1000581. [Google Scholar] [CrossRef]
- Kerimray, A.; Baimatova, N.; Ibragimova, O.P.; Bukenov, B.; Kenessov, B.; Plotitsyn, P.; Karaca, F. Assessing Air Quality Changes in Large Cities during COVID-19 Lockdowns: The Impacts of Traffic-Free Urban Conditions in Almaty, Kazakhstan. Sci. Total Environ. 2020, 730, 139179. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.Y.; Ma, J.; Boey, Y.C.F. Development of Nanomaterials for SALDI-MS Analysis in Forensics. Adv. Mater. 2012, 24, 4211–4216. [Google Scholar] [CrossRef]
- Qin, Z.; Hu, S.; Han, W.; Li, Z.; Xu, W.W.; Zhang, J.; Li, G. Tailoring Optical and Photocatalytic Properties by Single-Ag-Atom Exchange in Au13Ag12(PPh3)10Cl8 Nanoclusters. Nano Res. 2022, 15, 2971–2976. [Google Scholar] [CrossRef]
- Liu, Z.; Qin, Z.; Cui, C.; Luo, Z.; Yang, B.; Jiang, Y.; Lai, C.; Wang, Z.; Wang, X.; Fang, X.; et al. In-Situ Generation and Global Property Profiling of Metal Nanoclusters by Ultraviolet Laser Dissociation-Mass Spectrometry. Sci. China Chem. 2022, 65, 1196–1203. [Google Scholar] [CrossRef]
- Tanaka, K.; Waki, H.; Ido, Y.; Akita, S.; Yoshida, Y.; Yoshida, T. Protein and Polymer Analyses up to m/z 100,000 by Laser Ionization Time-of-flight Mass Spectrometry. Rapid Commun. Mass Spectrom. 1988, 2, 151–153. [Google Scholar] [CrossRef]
- Yagnik, G.B.; Hansen, R.L.; Korte, A.R.; Reichert, M.D.; Vela, J.; Lee, Y.J. Large Scale Nanoparticle Screening for Small Molecule Analysis in Laser Desorption Ionization Mass Spectrometry. Anal. Chem. 2016, 88, 8926–8930. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Scott, T.R.; Darrell, K.T.; Jia-Yuan, T.; Lin, H. Impacts of Surface Morphology on Ion Desorption and Ionization in Desorption Ionization on Porous Silicon (DIOS) Mass Spectrometry. J. Phys. Chem. C 2009, 113, 3076–3083. [Google Scholar] [CrossRef]
- Piszczek, P.; Radtke, A. Silver Nanoparticles Fabricated Using Chemical Vapor Deposition and Atomic Layer Deposition Techniques: Properties, Applications and Perspectives: Review. In Noble and Precious Metals–Properties, Nanoscale Effects and Applications; InTech: Hong Kong, China, 2018; pp. 187–213. [Google Scholar]
- Silina, Y.E.; Volmer, D.A. Nanostructured Solid Substrates for Efficient Laser Desorption/Ionization Mass Spectrometry (LDI-MS) of Low Molecular Weight Compounds. Analyst 2013, 138, 7053–7065. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Brandl, D.W.; Nordlander, P.; Halas, N.J. Plasmonic Nanostructures: Artificial Molecules. Acc. Chem. Res. 2007, 40, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, J.; Yao, S.; Guo, Y.; Xia, X. High-Sensitivity Matrix-Assisted Laser Desorption/Ionization Fourier Transform Mass Spectrometry Analyses of Small Carbohydrates and Amino Acids Using Oxidized Carbon Nanotubes Prepared by Chemical Vapor Deposition as Matrix. Anal. Chim. Acta 2007, 604, 158–164. [Google Scholar] [CrossRef]
- Hosu, I.S.; Sobaszek, M.; Ficek, M.; Bogdanowicz, R.; Drobecq, H.; Boussekey, L.; Barras, A.; Melnyk, O.; Boukherroub, R.; Coffinier, Y. Carbon Nanowalls: A New Versatile Graphene Based Interface for the Laser Desorption/Ionization-Mass Spectrometry Detection of Small Compounds in Real Samples. Nanoscale 2017, 9, 9701–9715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakai, R.; Ichikawa, T.; Kondo, H.; Ishikawa, K.; Shimizu, N.; Ohta, T.; Hiramatsu, M.; Hori, M. Effects of Carbon Nanowalls (Cnws) Substrates on Soft Ionization of Low-Molecular-Weight Organic Compounds in Surface-Assisted Laser Desorption/Ionization Mass Spectrometry (Saldi-Ms). Nanomaterials 2021, 11, 262. [Google Scholar] [CrossRef] [PubMed]
- Merino, J.P.; Serna, S.; Criado, A.; Centeno, A.; Napal, I.; Calvo, J.; Zurutuza, A.; Reichardt, N.; Prato, M. Mass Spectrometry of Carbohydrate-Protein Interactions on a Glycan Array Conjugated to CVD Graphene Surfaces. 2D Mater. 2020, 7, 024003. [Google Scholar] [CrossRef]
- Szlyk, E.; Piszczek, P.; Chaberski, M.; Goliński, A. Studies of Thermal Decomposition Process of Ag(I) Perfluorinated Carboxylates with Temperature Variable IR and MS. Polyhedron 2001, 20, 2853–2861. [Google Scholar] [CrossRef]
- Szłyk, E.; Piszczek, P.; Grodzicki, A.; Chaberski, M.; Goliński, A.; Szatkowski, J.; Błaszczyk, T. CVD of AgI Complexes with Tertiary Phosphines and Perfluorinated Carboxylates—A New Class of Silver Precursors. Chem. Vap. Depos. 2001, 7, 111–116. [Google Scholar] [CrossRef]
- Piszczek, P.; Szłyk, E.; Chaberski, M.; Taeschner, C.; Leonhardt, A.; Bała, W.; Bartkiewicz, K. Characterization of Silver Trimethylacetate Complexes with Tertiary Phosphines as CVD Precursors of Thin Silver Films. Chem. Vap. Depos. 2005, 11, 53–59. [Google Scholar] [CrossRef]
- Radtke, A.; Grodzicka, M.; Ehlert, M.; Muzioł, T.M.; Szkodo, M.; Bartmański, M.; Piszczek, P. Studies on Silver Ions Releasing Processes and Mechanical Properties of Surface-Modified Titanium Alloy Implants. Int. J. Mol. Sci. 2018, 19, 3962. [Google Scholar] [CrossRef] [Green Version]
- Patiny, L.; Borel, A. ChemCalc: A Building Block for Tomorrow’s Chemical Infrastructure. J. Chem. Inf. Model. 2013, 53, 1223–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Zhou, Y.; Liu, C.; Pei, C.; Shu, W.; Zhang, C.; Liu, L.; Zhou, L.; Wan, J. Design of Multi-Shelled Hollow Cr2O3 Spheres for Metabolic Fingerprinting. Angew. Chem. Int. Ed. 2021, 60, 12504–12512. [Google Scholar] [CrossRef] [PubMed]
- Pei, C.; Liu, C.; Wang, Y.; Cheng, D.; Li, R.; Shu, W.; Zhang, C.; Hu, W.; Jin, A.; Yang, Y.; et al. FeOOH@Metal–Organic Framework Core–Satellite Nanocomposites for the Serum Metabolic Fingerprinting of Gynecological Cancers. Angew. Chem. Int. Ed. 2020, 59, 10831–10835. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Shi, X.; Gurav, D.D.; Huang, L.; Su, H.; Li, K.; Niu, J.; Zhang, M.; Wang, Q.; Jiang, M.; et al. Metabolic Fingerprinting on Synthetic Alloys for Medulloblastoma Diagnosis and Radiotherapy Evaluation. Adv. Mater. 2020, 32, 2000906. [Google Scholar] [CrossRef] [PubMed]
- Mandal, G.; Moráň, L.; Pečinka, L.; Vaňhara, P.; Havel, J. Matrix Enrichment by Black Phosphorus Improves Ionization and Reproducibility of Mass Spectrometry of Intact Cells, Peptides, and Amino Acids. Sci. Rep. 2022, 12, 1175. [Google Scholar] [CrossRef]
- Li, Y.; Cao, X.; Zhan, L.; Xue, J.; Wang, J.; Xiong, C.; Nie, Z. Hot Electron Transfer Promotes Ion Production in Plasmonic Metal Nanostructure Assisted Laser Desorption Ionization Mass Spectrometry. Chem. Commun. 2018, 54, 10905–10908. [Google Scholar] [CrossRef]
- Zhao, J.; Nguyen, S.C.; Ye, R.; Ye, B.; Weller, H.; Somorjai, G.A.; Alivisatos, A.P.; Dean Toste, F. A Comparison of Photocatalytic Activities of Gold Nanoparticles Following Plasmonic and Interband Excitation and a Strategy for Harnessing Interband Hot Carriers for Solution Phase Photocatalysis. ACS Cent. Sci. 2017, 3, 482–488. [Google Scholar] [CrossRef] [Green Version]
- Ganteför, G.; Gausa, M.; Meiwes-Broer, K.H.; Lutz, H.O. Photoelectron Spectroscopy of Silver and Palladium Cluster Anions. Electron Delocalization versus, Localization. J. Chem. Soc. Faraday Trans. 1990, 86, 2483–2488. [Google Scholar] [CrossRef] [Green Version]
- Ng, K.M.; Chau, S.L.; Tang, H.W.; Wei, X.G.; Lau, K.C.; Ye, F.; Ng, A.M.C. Ion-Desorption Efficiency and Internal-Energy Transfer in Surface-Assisted Laser Desorption/Ionization: More Implication(s) for the Thermal-Driven and Phase-Transition-Driven Desorption Process. J. Phys. Chem. C 2015, 119, 23708–23720. [Google Scholar] [CrossRef]
- Sherrod, S.D.; Diaz, A.J.; Russell, W.K.; Cremer, P.S.; Russell, D.H. Silver Nanoparticles as Selective Ionization Probes for Analysis of Olefins by Mass Spectrometry. Anal. Chem. 2008, 80, 6796–6799. [Google Scholar] [CrossRef]
- Jin, J.M.; Choi, S.; Kim, Y.H.; Choi, M.H.; Kim, J.; Kim, S. Evaluation of Nanoporous Gold with Controlled Surface Structures for Laser Desorption Ionization (LDI) Analysis: Surface Area versus LDI Signal Intensity. J. Am. Soc. Mass Spectrom. 2012, 23, 1450–1453. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Precursor | Ag5(O2CC2F5)5(H2O)3 |
| Precursor weight (mg) | 2.5, 5, 10, 15, 35, 50, 70, 100 |
| Vaporization temperature (TV) (°C) | 230 |
| Carrier gas | Ar |
| Total reactor pressure (p) (mbar) | 3.0 |
| Substrate temperature (TD) (°C) | 290 |
| Substrates | stainless steel (H17) |
| Deposition time (min) | 60 |
| Sample heating time (min) | 30 (Ar/H2 (3:1%)) |
| Sample | Precursor Weight (mg) | Percentage Substrate Mass Increase after the CVD Process (wt.%) | AgPs Medium Grain Size (μm) |
|---|---|---|---|
| AgPs 0.20 | 100 | 0.20 | 0.7–2.8 ± 0.2–0.9 |
| AgPs 0.19 | 75 | 0.19 | 0.5–1.7 ± 0.2–1.0 |
| AgPs 0.17 | 50 | 0.17 | 0.2–0.7 ± 0.09–0.2 |
| AgPs 0.11 | 35 | 0.11 | 0.33 ± 0.09 |
| AgPs 0.06 | 15 | 0.06 | 0.24 ± 0.08 |
| AgPs 0.04 | 10 | 0.04 | 0.15 ± 0.05 |
| AgPs 0.03 | 5 | 0.03 | 0.05 ± 0.01 |
| AgPs 0.02 | 2.5 | ca. 0.02 | 0.15 ± 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagandykova, G.; Piszczek, P.; Radtke, A.; Mametov, R.; Pryshchepa, O.; Gabryś, D.; Kolankowski, M.; Pomastowski, P. Silver Nanostructured Substrates in LDI-MS of Low Molecular Weight Compounds. Materials 2022, 15, 4660. https://doi.org/10.3390/ma15134660
Sagandykova G, Piszczek P, Radtke A, Mametov R, Pryshchepa O, Gabryś D, Kolankowski M, Pomastowski P. Silver Nanostructured Substrates in LDI-MS of Low Molecular Weight Compounds. Materials. 2022; 15(13):4660. https://doi.org/10.3390/ma15134660
Chicago/Turabian StyleSagandykova, Gulyaim, Piotr Piszczek, Aleksandra Radtke, Radik Mametov, Oleksandra Pryshchepa, Dorota Gabryś, Mateusz Kolankowski, and Paweł Pomastowski. 2022. "Silver Nanostructured Substrates in LDI-MS of Low Molecular Weight Compounds" Materials 15, no. 13: 4660. https://doi.org/10.3390/ma15134660