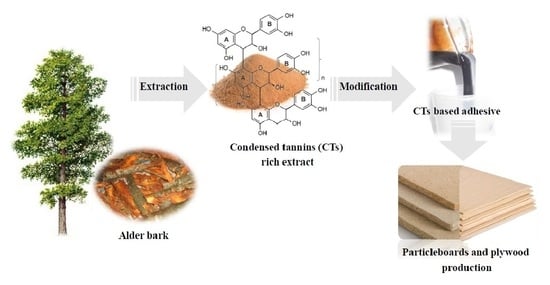
Eco-Friendly Adhesives Based on the Oligomeric Condensed Tannins-Rich Extract from Alder Bark for Particleboard and Plywood Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Alder Bark
2.1.2. Chemicals
2.1.3. Pine Particles for Particleboards
2.1.4. Veneer Sheets for Plywood
2.2. CTs-Rich Extract Isolation from Grey and Black Alder Bark
2.3. Determination of CTs Content in the Extract
2.4. Purification of CTS from Non-Tannin and Sugar
2.5. Determination of Carbohydrate Content in the Extract
2.6. Characterization of Purified CTs
2.7. Preparation and Characterization of CTs-Based Adhesive Systems
2.7.1. CTs–PEI Adhesive
2.7.2. CTs–PEI-ULEFR Adhesive
2.7.3. Synthesis of a PF Resin with Phenol Substitution by CTs-Rich Extract
2.8. Determination of pH
2.9. Determination of Adhesive System Viscosity and Gel Time
2.10. Determination of Dry Matter Content of Adhesive
2.11. Thermogravimetric Analysis
2.12. Manufacture and Testing of Wood Composites
2.12.1. Particleboard Production
- Mat formation: pressure 1.3 MPa, time 2–3 min;
- Cycle 1—pressure 3 MPa, time 5 min;
- Cycle 2—pressure 2 MPa, time 4 min;
- Cycle 3—pressure 1.5 MPa, time 5 min;
- Cycle 4—vapor pressure reduction at 0.2 MPa in 30 s.
2.12.2. Preparation of Plywood
- pressing temperature 140 °C;
- pressing pressure 2 MPa;
- holding time under pressure—10 min.
2.12.3. Preparation and Determination of the Adhesive Bonding Quality
2.12.4. Bending Test
2.13. Determination of Density
2.14. Determination of Formaldehyde Emission from Plywood Samples
2.15. Preparation and Determination of Particle Size of Extracted Alder Bark Residue
2.16. Statistical Analysis
3. Results and Discussion
3.1. Chemical Characterization of Hydrophilic Extracts and CTs from Alnus Incana and Alnus Glutinosa
3.2. Evaluation of Plywood Produced with CTs-PF Resin Subsection
3.3. Characterization of CTs-Based NAF Adhesive System
3.4. Particleboards Properties
3.5. Plywood Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mordor Intelligence. Available online: https://www.mordorintelligence.com/industry-reports/urea-formaldehyde-market (accessed on 12 April 2022).
- Qiao, W.; Li, S.; Xu, F. Preparation and Characterization of a Phenol-formaldehyde Resin Adhesive Obtained from Bio-ethanol Production Residue. Polym. Polym. Compos. 2016, 18, 99–105. [Google Scholar] [CrossRef]
- Sarika, P.R.; Nancarrow, P.; Khansaheb, A.; Ibrahim, T. Bio-based alternatives to phenol and formaldehyde for the production of resins. Polymers 2020, 12, 2237. [Google Scholar] [CrossRef] [PubMed]
- Asima, M.; Saba, N.; Jawaid, M.; Nasirc, M.; Pervaizd, M.; Alothman, O.Y. A Review on Phenolic Resin and its Composites. Curr. Anal. Chem. 2018, 14, 185–197. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Formaldehyde, 2-Butoxyethanol and 1-tert-Butoxypropanol-2-ol. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; World Health Organization: Lyon, France, 2006; pp. 39–325. [Google Scholar]
- Ghani, A.; Ashaari, Z.; Bawon, P.; Lee, S.H. Reducing formaldehyde emission of urea formaldehyde-bonded particleboard by addition of amines as formaldehyde scavenger. Build. Environ. 2018, 142, 188–194. [Google Scholar] [CrossRef]
- Kristak, L.; Antov, P.; Bekhta, P.; Lubis, M.A.R.; Iswanto, A.H.; Reh, R.; Sedliacik, J.; Savov, V.; Taghiyari, H.R.; Papadopoulos, A.N.; et al. Recent progress in ultra-low formaldehyde emitting adhesive systems and formaldehyde scavengers in wood-based panels: A review. Wood Mater. Sci. Eng. 2022, 1–20. [Google Scholar] [CrossRef]
- Kamperidou, V.; Barboutis, I. Bondability of Black locust (Robinia pseudoacacia) and Beech wood (Fagus sylvatica) with polyvinyl acetate and polyurethane adhesives. Maderas. Cienc. Tecnol. 2017, 19, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Kowaluk, G.; Fuczek, D. PVAc glue as a binding agent in particleboards. Drewno 2009, 182, 17–24. [Google Scholar]
- Ang, A.F.; Ashaari, Z.; Lee, S.H.; Tahir, P.M.; Halis, R. Lignin-based copolymer adhesives for composite wood panels—A review. Int. J. Adhes. Adhes. 2019, 95, 102408. [Google Scholar] [CrossRef]
- Luo, X.; Shuai, L. Lignin-Based Adhesives. In Green Adhesives: Preparation, Properties and Applications; Wiley: Hoboken, NJ, USA, 2020; pp. 25–56. [Google Scholar] [CrossRef]
- Larregle, A.; Chalapud, M.; Fangio, F.; Ciannamea, E.M.; Stefani, P.M.; Martucci, J.F.; Ruseckaite, R.A. Antifungal Soybean Protein Concentrate Adhesive as Binder of Rice Husk Particleboards. Polymers 2021, 13, 3540. [Google Scholar] [CrossRef]
- Chen, S.; Chen, Y.; Wang, Z.; Chen, H.; Fan, D. Renewable bio-based adhesive fabricated from a novel biopolymer and soy protein. RSC Adv. 2021, 11, 11724–11731. [Google Scholar] [CrossRef]
- Vnučec, D.; Kutnar, A.; Goršek, A. Soy-based adhesives for wood-bonding—A review. J. Adhes. Sci. Technol. 2016, 31, 910–931. [Google Scholar] [CrossRef]
- Sepahvand, S.; Doosthosseini, K.; Pirayesh, H.; Karamdoost Maryan, B. Supplementation of Natural Tannins as an Alternative to Formaldehyde in Urea and Melamine Formaldehyde Resins used in MDF Production. Drv. Ind. 2018, 69, 215–221. [Google Scholar] [CrossRef]
- Coppens, H.A.; Santana, N.A.E.; Pastore, F.J. Tannin formaldehyde adhesive for exterior-grade plywood and particleboard manufacture [Acacia mollissima, Rhizophora mangle]. For. Prod. J. 1980, 30, 38–42. [Google Scholar]
- Li, X.; Essawy, H.A.; Pizzi, A.; Delmotte, L.; Rode, K.; Le Nouen, D.; Fierro, V.; Celzard, A. Modification of tannin based rigid foams using oligomers of a hyperbranched poly (amine-ester). J. Polym. Res. 2012, 19, 21. [Google Scholar] [CrossRef]
- Yang, T.; Dong, M.; Cui, J.; Gan, L.; Han, S. Exploring the formaldehyde reactivity of tannins with different molecular weight distributions: Bayberry tannins and larch tannins. Holzforschung 2019, 74, 673–682. [Google Scholar] [CrossRef]
- Tupciauskas, R.; Rizhikovs, J.; Grinins, J.; Paze, A.; Andzs, M.; Brazdausks, P.; Puke, M.; Plavniece, A. Investigation of suberinic acids-bonded particleboard. Eur. Polym. J. 2019, 113, 176–182. [Google Scholar] [CrossRef]
- Braghiroli, F.L.; Amaral-Labat, G.; Boss, A.F.N.; Lacoste, C.; Pizzi, A. Tannin Gels and Their Carbon Derivatives: A Review. Biomolecules 2019, 9, 587. [Google Scholar] [CrossRef] [Green Version]
- Réh, R.; Krišták, L.; Sedliacik, J.; Bekhta, P.; Božiková, M.; Kunecová, D.; Vozárová, V.; Tudor, E.M.; Antov, P.; Savov, V. Utilization of Birch Bark as an Eco-Friendly Filler in Urea-Formaldehyde Adhesives for Plywood Manufacturing. Polymers 2021, 13, 511. [Google Scholar] [CrossRef]
- Kumar Das, A.; Nazrul Islam, M.; Omar Faruk, M.; Ashaduzzaman, M.; Dungani, R.; Rosamah, E.; Rumidatul, A. Hardwood Tannin: Sources, Utilizations, and Prospects. In Tannins—Structural Properties, Biological Properties and Current Knowledge; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef] [Green Version]
- De Hoyos-Martínez, P.L.; Merle, J.; Labidi, J.; Charrier—El Bouhtoury, F. Tannins extraction: A key point for their valorization and cleaner production. J. Clean. Prod. 2019, 206, 1138–1155. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Du, G. Applications of Tannin Resin Adhesives in the Wood Industry. In Tannins—Structural Properties, Biological Properties and Current Knowledge; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef] [Green Version]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saaed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Antov, P.; Savov, V.; Neykov, N. Sustainable bio-based adhesives for eco-friendly wood composites a review. Wood Res. 2020, 65, 51–62. [Google Scholar] [CrossRef]
- Kemppainen, K.; Siika-aho, M.; Pattathil, S.; Giovando, S.; Kruus, K. Spruce bark as an industrial source of condensed tannins and non-cellulosic sugars. Ind. Crop. Prod. 2014, 52, 158–168. [Google Scholar] [CrossRef]
- Lv, Q.; Luo, F.; Zhao, X.; Liu, Y.; Hu, G.; Sun, C. Identification of Proanthocyanidins from Litchi (Litchi chinensis Sonn.) Pulp by LC-ESI-Q-TOF-MS and Their Antioxidant Activity. PLoS ONE 2015, 10, e0120480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Telysheva, G.; Dizhbite, T.; Bikovens, O.; Ponomarenko, J.; Janceva, S.; Krasilnikova, J. Structure and antioxidant activity of diarylheptanoids extracted from bark of grey alder (Alnus incana) and potential of biorefinery-based bark processing of European trees. Holzforschung 2011, 65, 623–629. [Google Scholar] [CrossRef]
- Mosiewicki, M.A.; Aranguren, M.I.; Curvelo, A.A.S.; Borrajo, J. Effect of natural rubber on wood-reinforced tannin composites. J. Appl. Polym. Sci. 2007, 105, 1825–1832. [Google Scholar] [CrossRef]
- Li, K.; Geng, X.; Simonsen, J.; Karchesy, J. Novel wood adhesives from condensed tannins and polyethyleneimine. Int. J. Adhes. Adhes. 2004, 24, 327–333. [Google Scholar] [CrossRef]
- Prabhu, R.; Jagtap, R.; Digar, M. Study on incorporating wattle tannin in polyvinyl acetate emulsion and its effect on properties for wood bonding application. SN Appl. Sci. 2020, 2, 1722. [Google Scholar] [CrossRef]
- Janceva, S.; Dizhbite, T.; Telisheva, G.; Spulle, U.; Klavinsh, L.; Dzenis, M. Tannins of deciduous trees bark as a potential source for obtaining ecologically safe wood adhesives. In Proceedings of the 8th International Scientific and Practical Conference, Rezekne, Latvia, 20–22 June 2011; pp. 265–270. [Google Scholar]
- Janceva, S.; Andzs, M.; Tupciauskas, R.; Telysheva, G.; Dizhbite, T.; Dzenis, M. Condensed tannins rich grey alder bark extract potentials as a raw material for wood adhesive production. In Proceedings of the 12th Meeting of the Nurthern European Network for Wood Science and Engineering (WSE), Riga, Latvia, 12–13 September 2016; pp. 58–62. [Google Scholar]
- Janceva, S.; Andersone, A.; Lauberte, L.; Bikovens, O.; Nikolajeva, V.; Jashina, L.; Zaharova, N.; Telysheva, G.; Senkovs, M.; Rieksts, G.; et al. Sea buckthorn (Hippophae rhamnoides) waste biomass after harvesting as a source of valuable biologically active compounds with nutraceutical and antibacterial potential. Plants 2022, 11, 642. [Google Scholar] [CrossRef]
- Papadopoulou, E. Adhesives from Renewable Resources for Binding Wood-Based Panels. 2009. Available online: https://chimarhellas.com/wp-content/uploads/2021/06/ADHESIVES-FROM-RENEWABLE-RESOURCES-FOR-BINDING-WOOD-BASED-PANELS.pdf (accessed on 3 May 2022).
- Papadopoulou, E. The Challenge of Bio–Adhesives for the Wood Composite Industries. Available online: https://chimarhellas.com/publication/the-challenge-of-bio-adhesives-for-the-wood-composite-industries/ (accessed on 3 May 2022).
- EN 310:1993; Wood-Based Panels—Determination of Modulus of Elasticity in Bending and of Bending Strength. APA—The Engineered Wood Association: Tacoma, WA, USA, 1993.
- EN 314–1:2014; Plywood. Bonding Quality Test Methods. APA—The Engineered Wood Association: Tacoma, WA, USA, 2014.
- EN 326–1:1994; Sampling, Cutting and Inspection of Wood-Based Panel Products—Sampling and Cutting of Test Pieces and Expression of Test Results. ETSI: Sophia-Antipolis, France, 1994.
- EN 314-2:1993; Plywood. Bonding Quality Requirements. ETSI: Sophia-Antipolis, France, 1993.
- EN 319:1993; Particleboards and Fibreboards—Determination of Tensile Strength Perpendicular to the Plane of the Board. APA—The Engineered Wood Association: Tacoma, WA, USA, 1993.
- EN 323:1993; Wood-Based Panels. Determination of Density. ETSI: Sophia-Antipolis, France, 1993.
- JIS A 1460:2001; Building Boards Determination of Formaldehyde Emission–Desiccator Method. ETSI: Sophia-Antipolis, France, 2001.
- EN 717-2:1994; Wood-Based Panels—Determination of Formaldehyde Release—Part 2: Formaldehyde Release by the Gas Analysis Method. ISO: Geneva, Switzerland, 1994.
- Liu, Y.; Feng, S.; Song, L.; He, G.; Chen, M.; Huang, D. Secondary Metabolites in Durian Seeds: Oligomeric Proanthocyanidins. Molecules 2013, 18, 14172–14185. [Google Scholar] [CrossRef]
- Pinnataip, R.; Lee, B.P. Oxidation Chemistry of Catechol Utilized in Designing Stimuli-Responsive Adhesives and Antipathogenic Biomaterials. ACS Omega 2021, 6, 5113–5118. [Google Scholar] [CrossRef]
- Chemistry. Available online: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Map%3A_Organic_Chemistry_(Wade)/20%3A_Amines/20.06%3A_Reactions_of_Amines (accessed on 12 April 2022).
- Moubarik, A.; Pizzi, A.; Allal, A.; Charrier, F.; Charrier, B. Cornstarch and tannin in phenol–formaldehyde resins for plywood production. Ind. Crop. Prod. 2009, 30, 188–193. [Google Scholar] [CrossRef]
- EN 312:2010; Particleboards—Specifications. ISO: Geneva, Switzerland, 2010.
- Brosse, N.; Pizzi, A. Tannins for wood adhesives, foams and composites: Preparation, Characterization, and Testing. In Bio-Based Wood Adhesives; Routledge: Oxfordshire, UK, 2017; pp. 197–220. [Google Scholar]












| Shear Strength, fm (N/mm2) | Wood Failure, W (%) |
|---|---|
| 0.2 ≤ fv < 0.4 | ≥80 |
| 0.4 ≤ fv < 0.6 | ≥60 |
| 0.6 ≤ fv < 1.0 | ≥40 |
| 1.0 ≤ fv | No requirements set |
| Characteristics | Standard PF Resin | CTs (20%)-PF Resin | ||
|---|---|---|---|---|
| No treatment | ||||
| Shear strength, N/mm2 | 2.58 ± 0.23 | 2.14 | 2.06 ± 0.31 | 1.47 |
| Wood failure, % | 60 ± 2 | - | 64 ± 1 | - |
| Pre-treatment required by Class 1: immersion in water of 20 °C for 24 h | ||||
| Shear strength, N/mm2 | 1.45 ± 0.22 | 1.03 | 1.62 ± 0.32 | 1.02 |
| Wood failure, % | 88 ± 2 | - | 60 ± 1 | - |
| Pre-treatment required by Class 3: 4 h in boiling water–16 h drying at 60 °C–4 h in boiling water–1 h in cool water | ||||
| Shear strength, N/mm2 | 1.31 ± 0.16 | 1.01 | 1.14 ± 0.11 | 0.93 |
| Wood failure, % | 83 ± 2 | - | 37 ± 1 | - |
| Formaldehyde emission (based on the requirements of JIS A 1460:2001, mg/L | ||||
| 0.090 ± 0.008 | - | 0.047 ± 0.009 | - | |
| Adhesive System | MOR, N/mm2 | MOE, N/mm2 | IB, N/mm2 | |||
|---|---|---|---|---|---|---|
| CTs-rich extract from Alnus incana bark extracted by 50% EtOH (42% CTs on o.d. extract) | ||||||
| CTs 20% aqueous solution (10% of pinewood particles mass) | 5.3 ± 1.4 | 2.7 | 819 ± 180 | 479 | - | - |
| CTs 20% aqueous solution (20% of pinewood particles mass) | 13.2 ± 3.2 | 7.2 | 1305 ± 293 | 751 | - | - |
| CTs–PEI (10% of pinewood particles mass) | 5.0 ± 1.1 | 2.9 | 699 ± 232 | 261 | - | - |
| CTs–PEI (20% of pinewood particles mass) | 16.9 ± 1.1 | 14.8 | 1904 ± 297 | 1342 | - | - |
| CTs-rich extract from Alnus glutinosa bark extracted by 50% EtOH (46% CTs on o.d. extract) | ||||||
| CTs–PEI (10% ofpinewood particles mass) | 5.2 ± 1.2 | 2.9 | 682 ± 226 | 255 | - | - |
| CTs–PEI (20% of pinewood particles mass) | 16.0 ± 0.6 | 14.9 | 1914 ± 231 | 1477 | 0.45 ± 0.04 | 0.40 |
| CTs-rich extract from Alnus glutinosa bark extracted by water (24% CTs on o.d. extract) | ||||||
| CTs–PEI (10% of pinewood particles mass) | 4.8 ± 1.1 | 2.7 | 696 ± 227 | 267 | - | - |
| CTs–PEI (20% of pinewood particles mass) | 12.1 ± 0.8 | 10.6 | 1283 ± 216 | 875 | - | - |
| EN 312 P2 standard | - | 11 | - | 1800 | - | 0.40 |
| Adhesive Composition (w:w) | Viscosity, mPa·s, at 25 °C | MOE, N/mm2 | Shear Strength, N/mm2 | Density, kg/m3 | Moisture, % | ||
|---|---|---|---|---|---|---|---|
| Perpendicular to Wood Grains | Parallel to Wood Grains | After Immersion in Water at 20 °C (Class 1) | After Cyclic Treatment in Boiling Water (Class 2) | ||||
| ULEFR | 810 ± 30 | 1190 ± 160 | 16,270 ± 1800 | 2.28 ± 0.38 | 1.68 ± 0.30 | 666 | 8–9 |
| - | 888 | 12,868 | 1.56 | 1.11 | - | - | |
| (CTs–PEI): ULEFR = 2:8 | 124,680 ± 1800 | 1000 ± 150 | 14,610 ± 660 | 1.79 ± 0.38 | 1.56 ± 0.28 | 688 | 8–9 |
| - | 717 | 13,362 | 1.07 | 1.03 | - | - | |
| (CTs–PEI): ULEFR = 4:6 | >3,000,000 | 1070 ± 140 | 14,480 ± 880 | 1.84 ± 0.34 | 1.36 ± 0.27 | 722 | 8–9 |
| - | 805 | 12,817 | 1.20 | 0.85 | - | - | |
| (CTs–PEI): ULEFR = 1:1 | >3,000,000 | 1030 ± 110 | 14,085 ± 1300 | 2.04 ± 0.29 | 1.30 ± 0.32 | 702 | 8–9 |
| - | 822 | 11,628 | 1.49 | 0.70 | - | - | |
| (CTs–PEI): ULEFR = 6:4 | >3,000,000 | 1030 ± 120 | 13,980 ± 1730 | 1.45 ± 0.23 | 1.30 ± 0.29 | 644 | 8–9 |
| - | 803 | 10,710 | 1.02 | 0.75 | - | - | |
| (CTs–PEI): ULEFR = 8:2 | >3,000,000 | 1060 ± 200 | 11,720 ± 1380 | 0 | 0 | 736 | 8–9 |
| - | 682 | 9112 | - | - | - | - | |
| Adhesive Composition (w:w) | Pretreatment of Plywood Sample | Shear Strength, N/mm2 | |
|---|---|---|---|
| CTs–PEI-ULEFR (6:4) | After immersion in water at 20 °C (Class 1) | 1.45 ± 0.23 | 1.02 |
| CTs–PEI-ULEFR (6:4) + 10% filler | 1.62 ± 0.26 | 1.13 | |
| CTs–PEI-ULEFR (4:6) | After cyclic treatment in boiling water (Class 2) | 1.36 ± 0.27 | 0.85 |
| CTs–PEI-ULEF (4:6) + 10% filler | 1.40 ± 0.18 | 1.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janceva, S.; Andersone, A.; Spulle, U.; Tupciauskas, R.; Papadopoulou, E.; Bikovens, O.; Andzs, M.; Zaharova, N.; Rieksts, G.; Telysheva, G. Eco-Friendly Adhesives Based on the Oligomeric Condensed Tannins-Rich Extract from Alder Bark for Particleboard and Plywood Production. Materials 2022, 15, 3894. https://doi.org/10.3390/ma15113894
Janceva S, Andersone A, Spulle U, Tupciauskas R, Papadopoulou E, Bikovens O, Andzs M, Zaharova N, Rieksts G, Telysheva G. Eco-Friendly Adhesives Based on the Oligomeric Condensed Tannins-Rich Extract from Alder Bark for Particleboard and Plywood Production. Materials. 2022; 15(11):3894. https://doi.org/10.3390/ma15113894
Chicago/Turabian StyleJanceva, Sarmite, Anna Andersone, Uldis Spulle, Ramunas Tupciauskas, Electra Papadopoulou, Oskars Bikovens, Martins Andzs, Natalija Zaharova, Gints Rieksts, and Galina Telysheva. 2022. "Eco-Friendly Adhesives Based on the Oligomeric Condensed Tannins-Rich Extract from Alder Bark for Particleboard and Plywood Production" Materials 15, no. 11: 3894. https://doi.org/10.3390/ma15113894