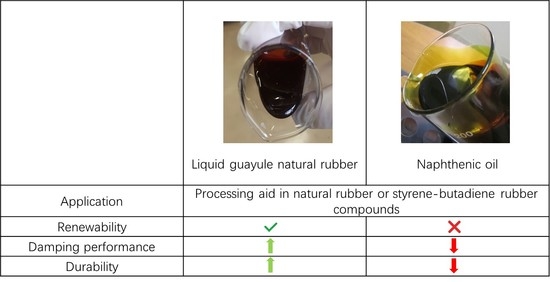
Liquid Guayule Natural Rubber, a Sustainable Processing Aid, Enhances the Processability, Durability and Dynamic Mechanical Properties of Rubber Composites
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Rheology Tests
3.2. Thermal Analysis
3.3. Dynamic Mechanical Analysis
3.4. Static and Dynamic Stiffness
3.5. Aged Mechanical Properties
3.6. Ozone Resistance
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Halász, I.Z.; Bárány, T. Novel Bifunctional Additive for Rubbers: Cyclic Butylene Terephthalate Oligomer. Period. Polytech. Mech. Eng. 2015, 59, 182–188. [Google Scholar] [CrossRef] [Green Version]
- Ortega, L.; Cerveny, S.; Sill, C.; Isitman, N.A.; Rodriguez-Garraza, A.L.; Meyer, M.; Westermann, S.; Schwartz, G.A. The effect of vulcanization additives on the dielectric response of styrene-butadiene rubber compounds. Polymer 2019, 172, 205–212. [Google Scholar] [CrossRef]
- Azeem, M.; Borg-Karlson, A.K.; Rajarao, G.K. Sustainable bio-production of styrene from forest waste. Bioresour. Technol. 2013, 144, 684–688. [Google Scholar] [CrossRef]
- Priyadarshan, P.M. Biology of Hevea Rubber; CABI: Wallingford, UK, 2011. [Google Scholar]
- Priyadarshan, P.M.; Hoa, T.T.T.; Huasun, H.; Street, T.; Chi, H.; City, M.; Postal, C. Yielding Potential of Rubber (Hevea brasiliensis) in Sub-Optimal Environments. J. Crop Improv. 2005, 14, 221–247. [Google Scholar] [CrossRef]
- Salvucci, M.E.; Coffelt, T.A.; Cornish, K. Improved methods for extraction and quantification of resin and rubber from guayule. Ind. Crops Prod. 2009, 30, 9–16. [Google Scholar] [CrossRef]
- Premadasa, R.B. ANRPC Releases Natural Rubber Trends & Statistics December 2020. Available online: http://www.anrpc.org/html/news-secretariat-details.aspx?ID=9&PID=39&NID=2271 (accessed on 12 January 2021).
- GlobalData Global Styrene-Butadiene-Rubber (SBR) Industry Outlook to 2025—Capacity and Capital Expenditure Forecasts with Details of All Active and Planned Plants. Available online: https://store.globaldata.com/report/global-styrene-butadiene-rubber-sbr-industry-outlook-to-2025-capacity-and-capital-expenditure-forecasts-with-details-of-all-active-and-planned-plants/ (accessed on 12 January 2021).
- Ramirez-Cadavid, D.A.; Cornish, K.; Michel, F.C. Taraxacum kok-saghyz (TK): Compositional analysis of a feedstock for natural rubber and other bioproducts. Ind. Crops Prod. 2017, 107, 624–640. [Google Scholar] [CrossRef]
- Ikeda, Y.; Junkong, P.; Ohashi, T.; Phakkeeree, T.; Sakaki, Y.; Tohsan, A.; Kohjiya, S.; Cornish, K. Strain-induced crystallization behaviour of natural rubbers from guayule and rubber dandelion revealed by simultaneous time-resolved WAXD/tensile measurements: Indispensable function for sustainable resources. RSC Adv. 2016, 6, 95601–95610. [Google Scholar] [CrossRef]
- Barrera, C.S.; Soboyejo, A.B.O.; Cornish, K. Quantification of the Contribution of Filler Characteristics To Natural Rubber Reinforcement Using Principal Component Analysis. Rubber Chem. Technol. 2017, 91, 79–96. [Google Scholar] [CrossRef]
- Barrera, C.S.; Cornish, K. Novel Mineral and Organic Materials from Agro-Industrial Residues as Fillers for Natural Rubber. ASTM Int. 2015, 23, 437–448. [Google Scholar] [CrossRef]
- Barrera, C.S.; Cornish, K. Processing and mechanical properties of natural rubber/waste-derived nano filler composites compared to macro and micro filler composites. Ind. Crops Prod. 2017, 107, 217–231. [Google Scholar] [CrossRef]
- Barrera, C.S.; Cornish, K. High performance waste-derived filler/carbon black reinforced guayule natural rubber composites. Ind. Crops Prod. 2016, 86, 132–142. [Google Scholar] [CrossRef] [Green Version]
- Rasutis, D.; Soratana, K.; McMahan, C.; Landis, A.E. A sustainability review of domestic rubber from the guayule plant. Ind. Crops Prod. 2015, 70, 383–394. [Google Scholar] [CrossRef]
- McMahan, C.; Kostyal, D.; Lhamo, D.; Cornish, K. Protein influences on guayule and Hevea natural rubber sol and gel. J. Appl. Polym. Sci. 2015, 132, 1–7. [Google Scholar] [CrossRef]
- Cornish, K.; Brichta, J.L.; Yu, P.; Wood, D.F.; Mcglothlin, M.W.; Martin, J.A. Guayule latex provides a solution for the critical demands of the non-allergenic medical products market. Ind. Crop. 2002, 12, 27–32. [Google Scholar]
- Moore, M. Ford, Cooper Make Inroads in Use of Guayule. Available online: http://www.rubbernews.com/article/20161123/NEWS/311149988/ford-cooper-make-inroads-in-use-of-guayule (accessed on 12 January 2021).
- Junkong, P.; Cornish, K.; Ikeda, Y. Characteristics of mechanical properties of sulphur cross-linked guayule and dandelion natural rubbers. RSC Adv. 2017, 7, 50739–50752. [Google Scholar] [CrossRef] [Green Version]
- Rensch, G.J.; Phillips, P.J.; Vatansever, N.; Gonzalez, V.A. The crystallization behavior of Cis-polyisoprenes extracted from the guayule plant. J. Polym. Sci. Part B Polym. Phys. 1986, 24, 1943–1959. [Google Scholar] [CrossRef]
- Li, J.; Isayev, A.I.; Ren, X.; Soucek, M.D. Toward Replacement of Petroleum Oils By Modified Soybean Oils in Elastomers. Rubber Chem. Technol. 2016, 89, 608–630. [Google Scholar] [CrossRef]
- Petrović, Z.S.; Ionescu, M.; Milić, J.; Halladay, J.R. Soybean Oil Plasticizers As Replacement of Petroleum Oil in Rubber. Rubber Chem. Technol. 2013, 86, 233–249. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhuang, T.; Shi, X.; Van Duin, M.; Zhao, S. Peroxide cross-linking of EPDM using moving die rheometer measurements. II: Effects of the process oils. RUBBER Chem. Technol. 2018, 91, 561–576. [Google Scholar] [CrossRef]
- EU Directive 2005/69/EC of the European Parliament and of the Council of 16 November 2005 amending for the 27th time Council Directive 76/769/EEC on the approximation of the laws, regulations and administrative provisions of the Member States relating to res. Off. J. Eur. Union 2005, L323, 51.
- Menon, A.R.R.; Pillai, C.K.S.; Nando, G.B. Cure characteristics and physicomechanical properties of natural rubber modified with phosphorylated cashew nut shell liquid prepolymer-A comparison with aromatic oil. J. Appl. Polym. Sci. 1999, 73, 813–818. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, Y.; Zhang, L.; Zhao, Y.; Vyzhimov, R.; Tan, T.; Fong, H. Investigation of Palm Oil as Green Plasticizer on the Processing and Mechanical Properties of Ethylene Propylene Diene Monomer Rubber. Ind. Eng. Chem. Res. 2016, 55, 2784–2789. [Google Scholar] [CrossRef]
- Raju, P.; Nandanan, V.; Kutty, S.K.N. A Study on the Use of Castor Oil as Plasticizer in Natural Rubber Compounds. J. Rubber Res. 2007, 23, 169–180. [Google Scholar] [CrossRef]
- Nandanan, V.; Joseph, R.; Francis, D.J. Linseed Oil as a Multipurpose Ingredient in NBR Vulcanizate. J. Elastomers Plast. 1996, 28, 326–334. [Google Scholar] [CrossRef]
- Saramolee, P.; Sahakaro, K.; Lopattananon, N.; Dierkes, W.K.; Noordermeer, J.W.M. Comparative properties of silica-and carbon black-reinforced natural rubber in the presence of epoxidized low molecular weight polymer. Rubber Chem. Technol. 2014, 87, 320–339. [Google Scholar] [CrossRef]
- Ren, X.; Barrera, C.S.; Tardiff, J.L.; Gil, A.; Cornish, K. Liquid guayule natural rubber, a renewable and crosslinkable processing aid in natural and synthetic rubber compounds. J. Clean. Prod. 2020, 276, 122933. [Google Scholar] [CrossRef]
- Cornish, K. Hypoallergenic Natural Rubber Products from Parthenum argentatum (Gray) and Other Non-Hevea Brasiliensis Species. US Patent 5,580,942, 3 December 1996. [Google Scholar]
- Wang, M. Effect of polymer-filler and filler-filler interactions on dynamic properties of filled vulcanizates. Rubber Chem. Technol. 1998, 71, 520–589. [Google Scholar] [CrossRef]
- Mohanty, T.R.; Bhandari, V.; Chandra, A.K.; Chattopadhyay, P.K.; Chattopadhyay, S. Role of calcium stearate as a dispersion promoter for new generation carbon black-organoclay based rubber nanocomposites for tyre application. Polym. Compos. 2013, 34, 214–224. [Google Scholar] [CrossRef]
- Ren, X.; Sancaktar, E. Use of fly ash as eco-friendly filler in synthetic rubber for tire applications. J. Clean. Prod. 2019, 206, 374–382. [Google Scholar] [CrossRef] [Green Version]
- Flory, P.J.; Rehner, J. Statistical Mechanics of Cross-Linked Polymer Networks I. Rubberlike Elasticity. J. Chem. Phys. 1943, 11, 512–520. [Google Scholar] [CrossRef]
- ASTM Standard D6814−02 Standard Test Method for Determination of Percent Devulcanization of Crumb Rubber Based on Crosslink Density. ASTM Int. 2014. Available online: https://www.astm.org/d6814-02r08.html (accessed on 12 January 2021).
- Marzocca, A.J. Evaluation of the polymer–solvent interaction parameter X for the system cured styrene butadiene rubber and toluene. Eur. Polym. J. 2007, 43, 2682–2689. [Google Scholar] [CrossRef]
- Kulkarni, P.P.; Jafvert, C.T. Solubility of C60 in Solvent Mixtures. Environ. Sci. Technol. 2008, 42, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Thaijaroen, W. Effect of tackifiers on mechanical and dynamic properties of carbon-black-filled NR vulcanizates. Polym. Eng. Sci. 2011, 51, 2465–2472. [Google Scholar] [CrossRef]
- Pazur, R.J.; Kennedy, T.A.C. Effect of Plasticizer Extraction By Jet Fuel on a Nitrile Hose Compound. Rubber Chem. Technol. 2015, 88, 324–342. [Google Scholar] [CrossRef]
- Rattanasom, N.; Saowapark, T.; Deeprasertkul, C. Reinforcement of natural rubber with silica/carbon black hybrid filler. Polym. Test. 2007, 26, 369–377. [Google Scholar] [CrossRef]
- Choi, S.-S. Influence of rubber composition on change of crosslink density of rubber vulcanizates with EV cure system by thermal aging. J. Appl. Polym. Sci. 2000, 75, 1378–1384. [Google Scholar] [CrossRef]
- Joseph, A.M.; George, B.; Madhusoodanan, K.N.; Alex, R. Current status of sulphur vulcanization and devulcanization chemistry: Process of vulcanization. Rubber Sci. 2015, 28, 82–121. [Google Scholar]
- Zhao, J.; Yang, R.; Iervolino, R.; Barbera, S. Changes of Chemical Structure and Mechanical Property Levels During Thermo-Oxidative Aging of Nbr. Rubber Chem. Technol. 2013, 86, 591–603. [Google Scholar] [CrossRef]
- Hamed, G.R.; Zhao, J. Tensile Behavior after Oxidative Aging of Gum and Black-Filled Vulcanizates of SBR and NR. Rubber Chem. Technol. 1999, 72, 721–730. [Google Scholar] [CrossRef]






















| Material | phr | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| HNR | 100 | 100 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| SBR | 0 | 0 | 0 | 100 | 100 | 100 | 0 | 0 | 0 |
| GNR | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 100 | 100 |
| Carbon black N330 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 |
| Sulfur | 4.5 | 4.5 | 4.5 | 4.5 | 4.5 | 4.5 | 4.5 | 4.5 | 4.5 |
| ZnO | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| TBBS | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Stearic acid | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 6PPD | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| NO | 0 | 20 | 0 | 0 | 20 | 0 | 0 | 20 | 0 |
| LGNR | 0 | 0 | 20 | 0 | 0 | 20 | 0 | 0 | 20 |
| Material | phr | |
|---|---|---|
| HNR | 100 | 100 |
| Sulfur | 4.5 | 4.5 |
| ZnO | 5 | 5 |
| TBBS | 1 | 1 |
| Stearic acid | 1 | 1 |
| 6PPD | 2 | 2 |
| NO | 0 | 10 |
| LGNR | 10 | 0 |
| Processing Aids | Glass Transition Temperature (°C) |
|---|---|
| NO | −54 |
| LGNR | −62 |
| Rubber Matrix | Processing Aids | tan δ at 0 °C | tan δ at 60 °C |
|---|---|---|---|
| HNR | N/A | 0.21 | 0.12 |
| HNR | 20 NO | 0.21 | 0.11 |
| HNR | 20 LGNR | 0.24 | 0.16 |
| SBR | N/A | 0.21 | 0.09 |
| SBR | 20 NO | 0.23 | 0.11 |
| SBR | 20 LGNR | 0.21 | 0.12 |
| GNR | N/A | 0.23 | 0.16 |
| GNR | 20 NO | 0.25 | 0.16 |
| GNR | 20 LGNR | 0.26 | 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, X.; Barrera, C.S.; Tardiff, J.L.; Gil, A.; Cornish, K. Liquid Guayule Natural Rubber, a Sustainable Processing Aid, Enhances the Processability, Durability and Dynamic Mechanical Properties of Rubber Composites. Materials 2022, 15, 3605. https://doi.org/10.3390/ma15103605
Ren X, Barrera CS, Tardiff JL, Gil A, Cornish K. Liquid Guayule Natural Rubber, a Sustainable Processing Aid, Enhances the Processability, Durability and Dynamic Mechanical Properties of Rubber Composites. Materials. 2022; 15(10):3605. https://doi.org/10.3390/ma15103605
Chicago/Turabian StyleRen, Xianjie, Cindy S. Barrera, Janice L. Tardiff, Andres Gil, and Katrina Cornish. 2022. "Liquid Guayule Natural Rubber, a Sustainable Processing Aid, Enhances the Processability, Durability and Dynamic Mechanical Properties of Rubber Composites" Materials 15, no. 10: 3605. https://doi.org/10.3390/ma15103605