Synthesis and Characterization of Mesoporous Aluminum Silicate and Its Adsorption for Pb (II) Ions and Methylene Blue in Aqueous Solution
Abstract
:1. Introduction
2. Materials and Methods
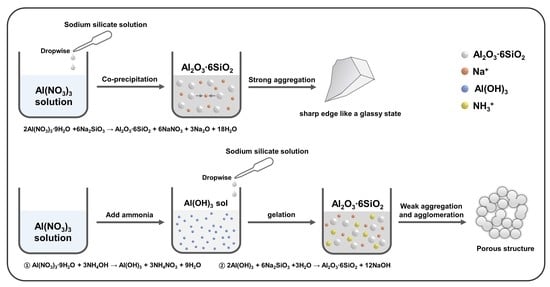
2.1. Synthesis of Aluminum Silicate Powder
2.2. Characterization
2.3. Pb (II) Ion Adsorption Test
2.4. Methylene Blue Adsorption Test
3. Results and Discussion
3.1. Characterization of the Synthesized Powder
3.2. Adsorption Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arshadi, M.; Faraji, A.R.; Mehravar, M. Dye removal from aqueous solution by cobalt-nano particles decorated aluminum silicate: Kinetic, thermodynamic and mechanism studies. J. Colloid Interface Sci. 2015, 440, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Gillis, P.L.; McInnis, R.; Salerno, J.; De Solla, S.R.; Servos, M.R.; Leonard, E.M. Municipal wastewater treatment plant effluent-induced effects on freshwater mussel populations and the role of mussel refugia in recolonizing an extirpated reach. Environ. Pollut. 2017, 225, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. Npj Clean Water 2021, 4, 1–15. [Google Scholar] [CrossRef]
- Wong, C.-W.; Barford, J.P.; Chen, G.; McKay, G. Kinetics and equilibrium studies for the removal of cadmium ions by ion exchange resin. J. Environ. Chem. Eng. 2014, 2, 698–707. [Google Scholar] [CrossRef]
- Yadav, V.B.; Gadi, R.; Kalra, S. Clay based nanocomposites for removal of heavy metals from water: A review. J. Environ. Manag. 2019, 232, 803–817. [Google Scholar] [CrossRef]
- Treto-Suárez, M.A.; Prieto-García, J.O.; Mollineda-Trujillo, Á.; Lamazares, E.; Hidalgo-Rosa, Y.; Mena-Ulecia, K. Kinetic study of removal heavy metal from aqueous solution using the synthetic aluminum silicate. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Arshadi, M.; Faraji, A.R.; Amiri, M.J. Modification of aluminum–silicate nanoparticles by melamine-based dendrimer l-cysteine methyl esters for adsorptive characteristic of Hg(II) ions from the synthetic and Persian Gulf water. Chem. Eng. J. 2015, 266, 345–355. [Google Scholar] [CrossRef]
- Hussain, A.; Madan, S.; Madan, R. Removal of Heavy Metals from Wastewater by Adsorption; IntechOpen: London, UK, 2021. [Google Scholar]
- Ince, M.; Kaplan İnce, O. An Overview of Adsorption Technique for Heavy Metal Removal from Water/Wastewater: A Critical Review. Int. J. Pure Appl. Sci. Technol. 2017, 3, 10–19. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, K.K.; Maseka, K.J.; Mbulo, M. Selected Adsorbents for Removal of Contaminants from Wastewater: Towards Engineering Clay Minerals. Open J. Appl. Sci. 2018, 8, 355–369. [Google Scholar] [CrossRef] [Green Version]
- Yuan, N.; Cai, H.; Liu, T.; Huang, Q.; Zhang, X. Adsorptive removal of methylene blue from aqueous solution using coal fly ash-derived mesoporous silica material. Adsorpt. Sci. Technol. 2019, 37, 333–348. [Google Scholar] [CrossRef] [Green Version]
- Renu; Agarwal, M.; Singh, K. Heavy metal removal from wastewater using various adsorbents: A review. J. Water Reuse Desalin. 2017, 7, 387–419. [Google Scholar] [CrossRef]
- Walcarius, A.; Mercier, L. Mesoporous organosilica adsorbents: Nanoengineered materials for removal of organic and inorganic pollutants. J. Mater. Chem. 2010, 20, 4478–4511. [Google Scholar] [CrossRef]
- Sari Yilmaz, M.; Karamahmut Mermer, N. Conversion of fly ashes from different regions to mesoporous silica: Effect of the mineralogical composition. J. Sol Gel Sci. Technol. 2016, 78, 239–247. [Google Scholar] [CrossRef]
- Belchinskaya, L.; Novikova, L.; Khokhlov, V.; Ly Tkhi, J. Contribution of Ion-Exchange and Non-Ion-Exchange Reactions to Sorption of Ammonium Ions by Natural and Activated Aluminosilicate Sorbent. J. Appl. Chem. 2013, 2013, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Krysztafkiewicz, A.; Lipska, L.K.; Ciesielczyk, F.; Jesionowski, T. Amorphous magnesium silicate—synthesis, physicochemical properties and surface morphology. Adv. Powder Technol. 2004, 15, 549–565. [Google Scholar] [CrossRef]
- Liu, M.; Hou, L.-A.; Xi, B.; Zhao, Y.; Xia, X. Synthesis, characterization, and mercury adsorption properties of hybrid mesoporous aluminosilicate sieve prepared with fly ash. Appl. Surf. Sci. 2013, 273, 706–716. [Google Scholar] [CrossRef] [Green Version]
- Hartati, H.; Purwaningsih, A.; Tjahjandarie, T.S.; Saputri, N.H.; Puspitasari, I.S.; Lamanele, C.N.; Sa’Adah, A.A.; Haque, A.S.; Mardho, D.Z. Synthesis of amorphous aluminosilicate from impure Indonesian kaolin. Open Chem. 2020, 18, 295–302. [Google Scholar] [CrossRef]
- Tokoro, C.; Suzuki, S.; Haraguchi, D.; Izawa, S. Silicate Removal in Aluminum Hydroxide Co-Precipitation Process. Materials 2014, 7, 1084–1096. [Google Scholar] [CrossRef]
- Alharthi, S.; El-Magied, M.O.A. Industrial by-product utilized synthesis of mesoporous aluminum silicate sorbent for thorium removal. Korean J. Chem. Eng. 2021, 38, 2365–2374. [Google Scholar] [CrossRef]
- Chen, X.; Mondal, P. Effects of NaOH amount on condensation mechanism to form aluminosilicate, case study of geopolymer gel synthesized via sol–gel method. J. Sol Gel Sci. Technol. 2020, 96, 589–603. [Google Scholar] [CrossRef]
- Dupuis, R.; Pellenq, R.; Champenois, J.-B.; Poulesquen, A. Dissociation Mechanisms of Dissolved Alkali Silicates in Sodium Hydroxide. J. Phys. Chem. C 2020, 124, 8288–8294. [Google Scholar] [CrossRef] [Green Version]
- Danks, A.E.; Hall, S.R.; Schnepp, Z. The evolution of ‘sol–gel’ chemistry as a technique for materials synthesis. Mater. Horiz. 2016, 3, 91–112. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Gao, Q.; Luo, W.; Zhou, Q.; Wang, H.; Yan, C.; Duan, P. Preparation, characterization and adsorption evaluation of spherical mesoporous Al-MCM-41 from coal fly ash. J. Taiwan Inst. Chem. Eng. 2015, 52, 147–157. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Subhani, T.; Wilayat Husain, S. Synthesis of silica nanoparticles from sodium silicate under alkaline conditions. J. Sol Gel Sci. Technol. 2016, 77, 753–758. [Google Scholar] [CrossRef]
- Akpomie, K.G.; Onoabedje, E.A.; Alumona, T.N.; Alum, O.L.; Okagu, O.D.; Ezeofor, C.C. Attenuation of Methylene Blue From Aqua-media on Acid Activated Montmorillonite of Nigerian Origin. J. Environ. Sci. Manag. 2017, 20, 17–27. [Google Scholar] [CrossRef]
- Buyang, S.; Yi, Q.; Cui, H.; Wan, K.; Zhang, S. Distribution and adsorption of metals on different particle size fractions of sediments in a hydrodynamically disturbed canal. Sci. Total Environ. 2019, 670, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Depasse, J. Coagulation of Colloidal Silica by Alkaline Cations: Surface Dehydration or Interparticle Bridging? J. Colloid Interface Sci. 1997, 194, 260–262. [Google Scholar] [CrossRef]
- Bae, E.; Lee, J.; Kim, Y.; Choi, K.; Yi, J. Sample Preparation and Analysis of Physico-Chemical Properties for Safety Assessment of Manufactured Nanomaterials. J. Korea Soc. Environ. Anal. 2009, 12, 59–73. [Google Scholar]
- Jung, S.J.; Lee, S.I.; Lim, H.M. Effect of the Concentration of Suspension and Electolyte on Zeta Potential. J. Korean Ceram. Soc. 2003, 40, 293–300. [Google Scholar]
- Wu, L.; Zhang, J.; Watanabe, W. Physical and chemical stability of drug nanoparticles. Adv. Drug Deliv. Rev. 2011, 63, 456–469. [Google Scholar] [CrossRef]
- Pan, M.-C.; Yang, Y.-X.; Ying, H.-P.; Jia, X.-C.; Chen, Y.-R.; Tang, Y. Effect of Variant Counterions on Stability and Particle Size of Silica Sol. Chin. J. Chem. 2007, 25, 1514–1521. [Google Scholar] [CrossRef]
- Mohammad Kooshafar, S.H.M. Influential Mechanisms and Potential Applications of Nano-Silicas in Cement Composites. Civ. Eng. Infrastruct. J. 2017, 50, 375–393. [Google Scholar] [CrossRef]
- Zeng, D.; Zhang, H.; Wang, B.; Sang, K.; Yang, J. Effect of Ammonia Concentration on Silica Spheres Morphology and Solution Hydroxyl Concentration in Stober Process. J. Nanosci. Nanotechnol. 2015, 15, 7407–7411. [Google Scholar] [CrossRef] [PubMed]
- Heitzinger, C.; Klimeck, G. Computational aspects of the three-dimensional feature-scale simulation of silicon-nanowire field-effect sensors for DNA detection. J. Comput. Electron. 2007, 6, 387–390. [Google Scholar] [CrossRef]
- Nilsson, S. Exploring Colloidal Silica Nanoparticles for Carbon Dioxide Capture and Storage; Chalmers University of Technology: Gothenburg, Sweden, 2016. [Google Scholar]
- Doroszkowski, A. 6—The physical chemistry of dispersion. In Paint and Surface Coatings, 2nd ed.; Lambourne, R., Strivens, T.A., Eds.; Woodhead Publishing: Sawston, UK, 1999; pp. 198–242. [Google Scholar]
- Ghernaout, D.; Elboughdiri, N.; Ghareba, S.; Salih, A. Coagulation Process for Removing Algae and Algal Organic Matter—An Overview. OAlib 2020, 7, 1–21. [Google Scholar] [CrossRef]
- Lowry, G.V.; Hill, R.J.; Harper, S.; Rawle, A.F.; Hendren, C.O.; Klaessig, F.; Nobbmann, U.; Sayre, P.; Rumble, J. Guidance to improve the scientific value of zeta-potential measurements in nanoEHS. Environ. Sci. Nano 2016, 3, 953–965. [Google Scholar] [CrossRef]
- Madadizadeh, A.; Sadeghein, A.; Riahi, S. The use of nanotechnology to prevent and mitigate fine migration: A comprehensive review. Rev. Chem. Eng. 2022, 38, 1–16. [Google Scholar] [CrossRef]
- Sogaard, C.; Funehag, J.; Abbas, Z. Silica sol as grouting material: A physio-chemical analysis. Nano Converg. 2018, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Tawari, S.L.; Koch, D.L.; Cohen, C. Electrical Double-Layer Effects on the Brownian Diffusivity and Aggregation Rate of Laponite Clay Particles. J. Colloid Interface Sci. 2001, 240, 54–66. [Google Scholar] [CrossRef]
- Ferrero, F. Adsorption of Methylene Blue on magnesium silicate: Kinetics, equilibria and comparison with other adsorbents. J. Environ. Sci. 2010, 22, 467–473. [Google Scholar] [CrossRef]
- Wang, W.; Tian, G.; Wang, D.; Zhang, Z.; Kang, Y.; Zong, L.; Wang, A. All-into-one strategy to synthesize mesoporous hybrid silicate microspheres from naturally rich red palygorskite clay as high-efficient adsorbents. Sci. Rep. 2016, 6, 39599. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Sample Notation | Ammonia Content (Conc.) |
|---|---|
| AS-0 | 0% |
| AS-2 | 2% |
| AS-4 | 4% |
| AS-6 | 6% |
| AS-8 | 8% |
| AS-10 | 10% |
| AS-12 | 12% |
| Wavenumber (cm−1) | Assignment | References |
|---|---|---|
| 455 | O-Si-O | [24] |
| 580 | Al-O6 | [19,20] |
| 715 | Al-O-Si | [19,20] |
| 1045 | Si-O-Si | [19,20,25] |
| 1400 | Al-OH | [19,20] |
| 1630 | H-O-H | [11,19,20] |
| 3440 | O-H | [11,19,20] |
| Sample | Particle Size (μm) | ||
|---|---|---|---|
| d10 | d90 | Mean | |
| AS-0 | 3.768 | 25.95 | 12.76 |
| AS-2 | 5.505 | 22.59 | 13.15 |
| AS-4 | 5.307 | 27.41 | 14.95 |
| AS-6 | 5.197 | 28.10 | 15.12 |
| AS-8 | 6.150 | 38.96 | 20.66 |
| AS-10 | 6.157 | 39.19 | 20.14 |
| AS-12 | 6.580 | 42.21 | 22.25 |
| Pb (II) Ions | |||
|---|---|---|---|
| Concentration (ppm) | Adsorption Capacity (mg/g) | Removal Efficiency (%) | |
| Initial | 520.4 | ||
| 0% | 413.2 | 26.8 | 20.6 |
| 4% | 68.4 | 113.0 | 86.9 |
| Methylene Blue | |||
| Concentration (ppm) | Adsorption Capacity (mg/g) | Removal Efficiency (%) | |
| Initial | 100 | ||
| 0% | 89.5 | 20.9 | 10.5 |
| 4% | 25.2 | 149.5 | 74.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, H.-S.; Kim, H.; Yoon, S.-Y. Synthesis and Characterization of Mesoporous Aluminum Silicate and Its Adsorption for Pb (II) Ions and Methylene Blue in Aqueous Solution. Materials 2022, 15, 3562. https://doi.org/10.3390/ma15103562
Jo H-S, Kim H, Yoon S-Y. Synthesis and Characterization of Mesoporous Aluminum Silicate and Its Adsorption for Pb (II) Ions and Methylene Blue in Aqueous Solution. Materials. 2022; 15(10):3562. https://doi.org/10.3390/ma15103562
Chicago/Turabian StyleJo, Hye-Soo, Hyeonjin Kim, and Seog-Young Yoon. 2022. "Synthesis and Characterization of Mesoporous Aluminum Silicate and Its Adsorption for Pb (II) Ions and Methylene Blue in Aqueous Solution" Materials 15, no. 10: 3562. https://doi.org/10.3390/ma15103562