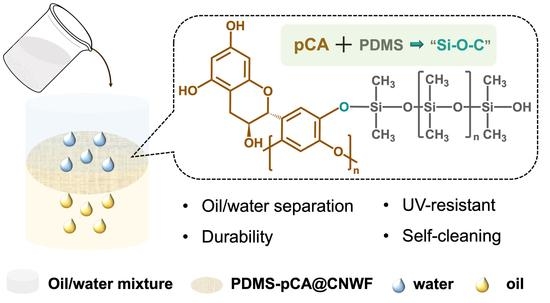
Superhydrophobic PDMS-pCA@CNWF Composite with UV-Resistant and Self-Cleaning Properties for Oil/Water Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Enzymatic Polymerization of Catechin
2.3. Antioxidant Activity of pCA
2.4. pCA-Modified CNWF
2.5. Preparation of Superhydrophobic PDMS-pCA@CNWF
2.6. Oil Adsorption Capacity and Oil/Water Separation Property of PDMS-pCA@CNWF
2.7. Mechanical Durability and Chemical Stability of PDMS-pCA@CNWF
2.8. Characterization
3. Results
3.1. Enzymatic Polymerization of Catechin
3.2. Preparation of Superhydrophobic PDMS-pCA@CNWF
3.3. Oil/water Separation Using PDMS-pCA@CNWF
3.4. Durability and Self-Cleaning Property of PDMS-pCA@CNWF
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, Q.; Zeng, G.; Yan, G. Self-cleaning photocatalytic MXene composite membrane for synergistically enhanced water treatment: Oil/water separation and dyes removal. Chem. Eng. J. 2022, 427, 131668. [Google Scholar] [CrossRef]
- Shen, Y.; Li, D.; Wang, L. Superelastic polyimide nanofiber-based aerogels modified with silicone nanofilaments for ultrafast oil/water separation. ACS Appl. Mater. Interfaces 2021, 13, 20489–20500. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Dunderdale, G.; England, M.; Hozumi, A. Oil/water separation techniques: A review of recent progresses and future directions. J. Mater. Chem. A 2017, 5, 16025–16058. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, G.; Luo, J. Novel membrane separation technologies and membrane processes. Front. Chem. Sci. Eng. 2021, 15, 717–719. [Google Scholar] [CrossRef]
- Guo, Z.; Long, B.; Gao, S. Carbon nanofiber based superhydrophobic foam composite for high performance oil/water separation. J. Hazard. Mater. 2021, 402, 123838. [Google Scholar] [CrossRef]
- Gou, X.; Zhang, Y.; Long, L. Superhydrophilic and underwater superoleophobic cement-coated mesh for oil/water separation by gravity. Colloids Surf. A Physicochem. Eng. Asp. 2020, 605, 125338. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, N.; Cao, Y.; Lin, X.; Liu, Y.; Feng, L. Superwetting porous materials for wastewater treatment: From immiscible oil/water mixture to emulsion separation. Adv. Mater. Interfaces 2017, 4, 1600029. [Google Scholar] [CrossRef]
- Yan, S.; Li, Y.; Xie, F. Environmentally safe and porous MS@TiO2@PPy monoliths with superior visible-light photocatalytic properties for rapid oil–water separation and water purification. ACS Sustain. Chem. Eng. 2020, 8, 5347–5359. [Google Scholar] [CrossRef]
- Chen, C.; Weng, D.; Mahmood, A. Separation mechanism and construction of surfaces with special wettability for oil/water separation. ACS Appl. Mater. Interfaces 2019, 11, 11006–11027. [Google Scholar] [CrossRef]
- Zhao, S.; Liang, Y.; Yang, Y. A robust surface with superhydrophobicity and underwater superoleophobicity for on-demand oil/water separation. Nanoscale 2021, 13, 15334–15342. [Google Scholar] [CrossRef]
- Padaki, M.; Murali, R.; Abdullah, M. Membrane technology enhancement in oil–water separation. A review. Desalination 2015, 357, 197–207. [Google Scholar] [CrossRef]
- Junaidi, N.; Othman, N.; Fuzil, N. Recent development of graphene oxide-based membranes for oil–water separation: A review. Sep. Purif. Technol. 2021, 258, 118000. [Google Scholar] [CrossRef]
- Zhan, H.; Zuo, T.; Tao, R. Robust tunicate cellulose nanocrystal/palygorskite nanorod membranes for multifunctional oil/water emulsion separation. ACS Sustain. Chem. Eng. 2018, 6, 10833–10840. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, J.; Zhang, F. Zwitterionic nanohydrogel grafted PVDF membranes with comprehensive antifouling property and superior cycle stability for oil-in-water emulsion separation. Adv. Funct. Mater. 2018, 28, 1804121. [Google Scholar] [CrossRef]
- Ou, J.; Hu, W.; Xue, M. Superhydrophobic surfaces on light alloy substrates fabricated by a versatile process and their corrosion protection. ACS Appl. Mater. Interfaces 2013, 5, 3101–3107. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Ding, Y.; Zhang, M.; Gao, S.; Li, Y.; Huang, C.; Fu, G. Nature-inspired chemistry toward hierarchical superhydrophobic, antibacterial and biocompatible nanofibrous membranes for effective UV-shielding, self-cleaning and oil–water separation. J. Hazard. Mater. 2020, 384, 121476. [Google Scholar] [CrossRef]
- Yuan, D.; Zhang, T.; Guo, Q.; Qiu, F.; Yang, D.; Ou, Z. Superhydrophobic hierarchical biomass carbon aerogel assembled with TiO2 nanorods for selective immiscible oil/water mixture and emulsion separation. Ind. Eng. Chem. Res. 2018, 57, 14758–14766. [Google Scholar] [CrossRef]
- Boinovich, L.; Emelyanenko, A.; Modestov, A. Synergistic effect of superhydrophobicity and oxidized layers on corrosion resistance of aluminum alloy surface textured by nanosecond laser treatment. ACS Appl. Mater. Interfaces 2015, 7, 19500–19508. [Google Scholar] [CrossRef] [Green Version]
- Nayak, K.; Tripathi, B. Molecularly grafted PVDF membranes with in-air superamphiphilicity and underwater superoleophobicity for oil/water separation. Sep. Purif. Technol. 2021, 259, 118068. [Google Scholar] [CrossRef]
- Goh, S.; Matsuura, T.; Ismail, A. Recent trends in membranes and membrane processes for desalination. Desalination 2016, 391, 43–60. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Mei, C. Wood-inspired anisotropic cellulose nanofibril composite sponges for multifunctional applications. ACS Appl. Mater. Interfaces 2020, 12, 35513–35522. [Google Scholar] [CrossRef]
- Abdelhamid, H.; Mathew, A. Cellulose-zeolitic imidazolate frameworks (CelloZIFs) for multifunctional environmental remediation: Adsorption and catalytic degradation. Chem. Eng. J. 2021, 426, 131733. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Liu, G. Rapid and efficient separation of oil from oil-in-water emulsions using a Janus cotton fabric. Angew. Chem. 2016, 128, 1313–1316. [Google Scholar] [CrossRef]
- Pi, P.; Hou, K.; Wen, X. A facile one-step fabrication of robust superhydrophobic/superoleophilic cotton fabric using a crosslinkable POSS-containing fluorinated copolymer. Prog. Org. Coat. 2016, 101, 522–529. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Yang, C. Nanospherical carbon nitride frameworks with sharp edges accelerating charge collection and separation at a soft photocatalytic interface. Adv. Mater. 2014, 26, 4121–4126. [Google Scholar] [CrossRef]
- Wei, P.; Lou, H.; Xu, X. Preparation of PP non-woven fabric with good heavy metal adsorption performance via plasma modification and graft polymerization. Appl. Surf. Sci. 2021, 539, 148195. [Google Scholar] [CrossRef]
- Jalvo, B.; Aguilar, A.; Ruiz, M. Water filtration membranes based on non-woven cellulose fabrics: Effect of nanopolysaccharide coatings on selective particle rejection, antifouling, and antibacterial properties. Nanomaterials 2021, 11, 1752. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, X.; Yan, L. Biomimetic nanoparticle-engineered superwettable membranes for efficient oil/water separation. J. Membr. Sci. 2021, 618, 118525. [Google Scholar] [CrossRef]
- Suner, S.; Sahiner, M.; Mohapatra, S. Degradable poly (catechin) nanoparticles as a versatile therapeutic agent. Int. J. Polym. Mater. 2021, 2021, 1–12. [Google Scholar] [CrossRef]
- Kurisawa, M.; Chung, J.; Uyama, H. Laccase-catalyzed synthesis and antioxidant property of poly(catechin). Macromol. Biosci. 2003, 3, 758–764. [Google Scholar] [CrossRef]
- Wen, H.; Hsu, Y.; Asoh, T. Poly(vinyl alcohol)-based composite film with Ag-immobilized TEMPO-oxidized nano-tea cellulose for improving photocatalytic performance. J. Mater. Sci. 2021, 56, 12224–12237. [Google Scholar] [CrossRef]
- Wang, L.; Dai, S.; Liu, X. A ternary system oleophilic–hydrophobic membrane prepared by electrospinning for efficient gravity-driven oil–water separation. SN Appl. Sci. 2019, 1, 797. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.; Asahara, H.; Hsu, Y. Surface modification of polycarbonate using the light-activated chlorine dioxide radical. Appl. Surf. Sci. 2020, 530, 147202. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, T.; Mkhize, N. GaS: WS2 heterojunctions for ultrathin two-dimensional photodetectors with large linear dynamic range across broad wavelengths. ACS Nano 2021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xue, J.; Yang, X. From plant phenols to novel bio-based polymers. Prog. Polym. Sci. 2022, 125, 101473. [Google Scholar] [CrossRef]
- Biao, L.; Tan, S.; Meng, Q. Green synthesis, characterization and application of proanthocyanidins-functionalized gold nanoparticles. Nanomaterials 2018, 8, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurisawa, M.; Chung, J.; Kim, Y. Amplification of antioxidant activity and xanthine oxidase inhibition of catechin by enzymatic polymerization. Biomacromolecules 2003, 4, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Ouyang, Y.; Kong, Y. Catechin inhibits the release of advanced glycation end products during glycated bovine serum albumin digestion and corresponding mechanisms in vitro. J. Agric. Food Chem. 2021, 69, 8807–8818. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wen, F.; Liu, Z. Hybrid siloxane-epoxy coating reinforced by worm-like graphene oxide with improved mechanical properties and corrosion resistance. Mater. Des. 2021, 207, 109852. [Google Scholar] [CrossRef]
- Zhang, B.; Duan, J.; Huang, Y. Double layered superhydrophobic PDMS-Candle soot coating with durable corrosion resistance and thermal-mechanical robustness. J. Mater. Sci. Technol. 2021, 71, 1–11. [Google Scholar] [CrossRef]
- Bauman, L.; Wen, Q.; Sameoto, D. Durable poly (N-isopropylacrylamide) grafted PDMS micropillared surfaces for temperature-modulated wetting. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125901. [Google Scholar] [CrossRef]
- Tantraviwat, D.; Ngamyingyoud, M.; Sripumkhai, W. Tuning the dielectric constant and surface engineering of a BaTiO3/Porous PDMS composite film for enhanced triboelectric nanogenerator output performance. ACS Omega 2021, 6, 29765–29773. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luo, Z.; Li, G. Solvent-free, ultrafast and ultrathin PDMS coating triggered by plasma for molecule separation and release. Green Chem. 2021, 23, 4181–4190. [Google Scholar] [CrossRef]
- Lisperguer, J.; Saravia, Y.; Vergara, E. Structure and thermal behavior of tannins from Acacia dealbata bark and their reactivity toward formaldehyde. J. Chil. Chem. Soc. 2016, 61, 3188–3190. [Google Scholar] [CrossRef] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, H.; Hsu, Y.-I.; Uyama, H. Superhydrophobic PDMS-pCA@CNWF Composite with UV-Resistant and Self-Cleaning Properties for Oil/Water Separation. Materials 2022, 15, 376. https://doi.org/10.3390/ma15010376
Wen H, Hsu Y-I, Uyama H. Superhydrophobic PDMS-pCA@CNWF Composite with UV-Resistant and Self-Cleaning Properties for Oil/Water Separation. Materials. 2022; 15(1):376. https://doi.org/10.3390/ma15010376
Chicago/Turabian StyleWen, Hanyu, Yu-I Hsu, and Hiroshi Uyama. 2022. "Superhydrophobic PDMS-pCA@CNWF Composite with UV-Resistant and Self-Cleaning Properties for Oil/Water Separation" Materials 15, no. 1: 376. https://doi.org/10.3390/ma15010376