Polypyrrole with Phosphor Tungsten Acid and Carbide-Derived Carbon: Change of Solvent in Electropolymerization and Linear Actuation
Abstract
:1. Introduction
2. Materials and methods
2.1. Materials
2.2. Electropolymerization
2.3. Linear Actuation Measurements
2.4. Characterization
3. Results and Discussions
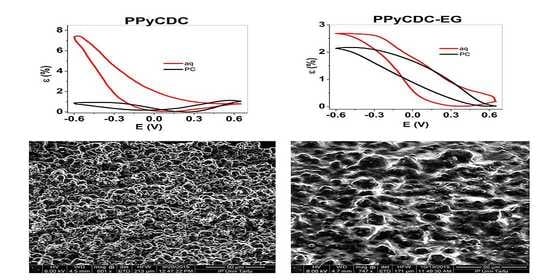
3.1. Electropolymerization, SEM, EDX and Electronic Conductivities
3.2. Linear Actuation
3.2.1. Cyclic Voltammetry
3.2.2. Square Potential Steps
3.3. Energy Storage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jager, E.W.H.; Smela, E.; Ingana, O. Microfabricating Conjugated Polymer Actuators. Science 2000, 290, 1540–1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jager, E.W.H.; Smela, E.; Inganäs, O.; Lundström, I. Polypyrrole microactuators. Synth. Met. 1999, 102, 1309–1310. [Google Scholar] [CrossRef] [Green Version]
- Jager, E.W.H. Microrobots for Micrometer-Size Objects in Aqueous Media: Potential Tools for Single-Cell Manipulation. Science 2000, 288, 2335–2338. [Google Scholar] [CrossRef] [PubMed]
- Smela, E. Conjugated polymer actuators for biomedical applications. Adv. Mater. 2003, 15, 481–494. [Google Scholar] [CrossRef]
- Svennersten, K.; Berggren, M.; Richter-Dahlfors, A.; Jager, E.W.H. Mechanical stimulation of epithelial cells using polypyrrole microactuators. Lab. Chip. 2011, 19, 3287–3293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maziz, A.; Concas, A.; Khaldi, A.; Stålhand, J.; Persson, N.-K.; Jager, E.W.H. Knitting and weaving artificial muscles. Sci. Adv. 2017, 3, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pope, M.T.; Müller, A. Polyoxometalate Chemistry: An Old Field with New Dimensions in Several Disciplines. Angew. Chem. Int. Ed. English 1991, 30, 34–48. [Google Scholar] [CrossRef]
- Zondaka, Z.; Kesküla, A.; Tamm, T.; Kiefer, R. Polypyrrole linear actuation tuned by phosphotungstic acid. Sens. Actuators B Chem. 2017, 247. [Google Scholar] [CrossRef] [Green Version]
- Zondaka, Z.; Valner, R.; Aabloo, A.; Tamm, T.; Kiefer, R. Embedded Carbide-derived Carbon (CDC) particles in polypyrrole (PPy) for linear actuator. In Proceedings of the SPIE: The International Society for Optical Engineering, Las Vegas, NV, USA, 20–24 March 2016; Volume 9798, p. 97981H. [Google Scholar]
- Khuyen, N.Q.; Kiefer, R.; Zondaka, Z.; Anbarjafari, G.; Peikolainen, A.; Otero, T.F.; Tamm, T. Multifunctionality of Polypyrrole Polyethyleneoxide Composites: Concurrent Sensing, Actuation and. Polymers 2020, 12, 2060. [Google Scholar] [CrossRef] [PubMed]
- Aydemir, N.; Kilmartin, P.A.; Travas-Sejdic, J.; Kesküla, A.; Peikolainen, A.L.; Parcell, J.; Harjo, M.; Aabloo, A.; Kiefer, R. Electrolyte and solvent effects in PPy/DBS linear actuators. Sens. Actuators B Chem. 2015, 216, 24–32. [Google Scholar] [CrossRef]
- Kiefer, R.; Martinez, J.G.; Kesküla, A.; Anbarjafari, G.; Aabloo, A.; Otero, T.F. Polymeric actuators: Solvents tune reaction-driven cation to reaction-driven anion actuation. Sens. Actuators B Chem. 2016, 233, 461–469. [Google Scholar] [CrossRef]
- Zondaka, Z.; Harjo, M.; Khorram, M.S.; Rasti, P.; Tamm, T.; Kiefer, R. Polypyrrole/carbide-derived carbon composite in organic electrolyte: Characterization as a linear actuator. React. Funct. Polym. 2018, 131, 414–419. [Google Scholar] [CrossRef]
- Khuyen, N.Q.; Zondaka, Z.; Harjo, M.; Torop, J.; Tamm, T.; Kiefer, R. Comparative Analysis of Fluorinated Anions for Polypyrrole Linear Actuator Electrolytes. Polymers 2019, 11, 849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiefer, R.; Chu, S.Y.; Kilmartin, P.A.; Bowmaker, G.A.; Cooney, R.P.; Travas-Sejdic, J. Mixed-ion linear actuation behaviour of polypyrrole. Electrochim. Acta 2007, 52, 2386–2391. [Google Scholar] [CrossRef]
- Gu, Y.; Qiao, Y.; Meng, Y.; Yu, M.; Zhang, B.; Li, J. One-step synthesis of well-dispersed polypyrrole copolymers under gamma-ray irradiation. Polym. Chem. 2021, 12, 645–649. [Google Scholar] [CrossRef]
- Yan, B.; Wu, Y.; Guo, L.; Yan, B.; Wu, Y.; Guo, L. Recent Advances on Polypyrrole Electroactuators. Polymers 2017, 9, 446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, H.K.; Lee, S.O.; Song, K.J.; Kim, S.G.; Kim, K.H. Synthesis and properties of soluble polypyrrole doped with dodecylbenzenesulfonate and combined with polymeric additive poly(ethylene glycol). J. Appl. Polym. Sci. 2005, 97, 1170–1175. [Google Scholar] [CrossRef]
- Nateghi, M.R.; Borhani zarandi, M.; Fathi, A. Structural and morphological aspects considerations of bilayers actuators based on polypyrrole/polyethylene glycole composites. Polym. Sci. Ser. A 2014, 56, 623–629. [Google Scholar] [CrossRef]
- Popescu, S.; Pirvu, C.; Mindroiu, M.; Demetrescu, I. Enhancing the stability of PPy film on Ti by PEG incorporation. Mol. Cryst. Liq. Cryst. 2010, 522, 125–135. [Google Scholar] [CrossRef]
- Pirvu, C.; Manole, C.C.; Stoian, A.B.; Demetrescu, I. Understanding of electrochemical and structural changes of polypyrrole/polyethylene glycol composite films in aqueous solution. Electrochim. Acta 2011, 56, 9893–9903. [Google Scholar] [CrossRef]
- Harjo, M.; Tamm, T.; Anbarjafari, G.; Kiefer, R. Hardware and Software Development for Isotonic Strain and Isometric Stress Measurements of Linear Ionic Actuators. Polymers 2019, 11, 1054. [Google Scholar] [CrossRef] [Green Version]
- Suárez, I.J.; Otero, T.F.; Márquez, M. Diffusion coefficients in swelling polypyrrole: ESCR and cottrell models. J. Phys. Chem. B 2005, 109, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Otero, T.F.; Martinez, J.G. Activation energy for polypyrrole oxidation: Film thickness influence. J. Solid State Electrochem. 2011, 15, 1169–1178. [Google Scholar] [CrossRef]
- Zondaka, Z.; Harjo, M.; Khan, A.; Khanh, T.T.; Tamm, T.; Kiefer, R. Optimal phosphotungstinate concentration for polypyrrole linear actuation and energy storage. Multifunct. Mater. 2018, 1, 14003. [Google Scholar] [CrossRef]
- Kim, Y.; Shanmugam, S. Polyoxometalate-reduced graphene oxide hybrid catalyst: Synthesis, structure, and electrochemical properties. ACS Appl. Mater. Interfaces 2013, 5, 12197–12204. [Google Scholar] [CrossRef]
- Zondaka, Z.; Valner, R.; Tamm, T.; Aabloo, A.; Kiefer, R. Carbide-derived carbon in polypyrrole changing the elastic modulus with a huge impact on actuation. RSC Adv. 2016, 6, 26380–26385. [Google Scholar] [CrossRef] [Green Version]
- Torop, J.; Aabloo, A.; Jager, E.W.H. Novel actuators based on polypyrrole/carbide-derived carbon hybrid materials. Carbon N. Y. 2014, 80, 387–395. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, Y.; Tazawa, S.; Fujii, Y.; Harada, I. Raman spectra of polypyrrole and its 2,5-13C-substituted and C-deuterared analogues in doped and undoped states. Synth. Met. 1988, 24, 329–341. [Google Scholar] [CrossRef]
- Zou, X.; Shen, Y.; Peng, Z.; Zhang, L.; Bi, L.; Wang, Y.; Dong, S. Preparation of a phosphopolyoxomolybdate P2Mo18O 626- doped polypyrrole modified electrode and its catalytic properties. J. Electroanal. Chem. 2004, 566, 63–71. [Google Scholar] [CrossRef]
- Crowley, K.; Cassidy, J. In situ resonance Raman spectroelectrochemistry of polypyrrole doped with dodecylbenzenesulfonate. J. Electroanal. Chem. 2003, 547, 75–82. [Google Scholar] [CrossRef]
- Demoustier-Champagne, S.; Stavaux, P.Y. Effect of electrolyte concentration and nature on the morphology and the electrical properties of electropolymerized polypyrrole nanotubules. Chem. Mater. 1999, 11, 829–834. [Google Scholar] [CrossRef]
- Matkovic, S.R.; Briand, L.E.; Bañares, M.Á. Investigation of the thermal stability of phosphotungstic Wells-Dawson heteropoly-acid through in situ Raman spectroscopy. Mater. Res. Bull. 2011, 46, 1946–1948. [Google Scholar] [CrossRef]
- Dash, R.K.; Nikitin, A.; Gogotsi, Y. Microporous carbon derived from boron carbide. Microporous Mesoporous Mater. 2004, 72, 203–208. [Google Scholar] [CrossRef]
- Liu, Y.C.; Hwang, B.J. Identification of oxidized polypyrrole on Raman spectrum. Synth. Met. 2000, 113, 203–207. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.; Zhu, J.; Ren, P. Electrochemical in situ polymerization of reduced graphene oxide/polypyrrole composite with high power density. J. Power Sources 2012, 208, 138–143. [Google Scholar] [CrossRef]
- Chen, F.; Shi, G.; Fu, M.; Qu, L.; Hong, X. Raman spectroscopic evidence of thickness dependence of the doping level of electrochemically deposited polypyrrole film. Synth. Met. 2003, 132, 125–132. [Google Scholar] [CrossRef]
- Wu, F.; Chen, J.; Li, L.; Zhao, T.; Liu, Z.; Chen, R. Polyethylene-glycol-doped polypyrrole increases the rate performance of the cathode in lithium-sulfur batteries. ChemSusChem 2013, 6, 1438–1444. [Google Scholar] [CrossRef]
- Krishnan, K.; Krishnan, R.S. Raman and infrared spectra of ethylene glycol. Proc. Indian Acad. Sci. Sect. A 1966, 64, 111–122. [Google Scholar] [CrossRef]
- Kivilo, A.; Zondaka, Z.; Kesküla, A.; Rasti, P.; Tamm, T.; Kiefer, R. Electro-chemo-mechanical deformation properties of polypyrrole/dodecylbenzenesulfate linear actuators in aqueous and organic electrolyte. RSC Adv. 2016, 6, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Kiefer, R.; Kilmartin, P.A.; Bowmaker, G.A.; Cooney, R.P.; Travas-Sejdic, J. Mixed-ion linear actuation of PPy and PEDOT in propylene carbonate-triflate electrolytes. Electroact. Polym. Actuators Devices 2007 2007, 6524, 65240U. [Google Scholar] [CrossRef]
- Torop, J.; Arulepp, M.; Leis, J.; Punning, A.; Johanson, U.; Palmre, V.; Aabloo, A. Nanoporous carbide-derived carbon material-based linear actuators. Materials 2010, 3, 9–25. [Google Scholar] [CrossRef] [Green Version]
- Pomerantz, Z.; Zaban, A.; Ghosh, S.; Lellouche, J.P.; Garcia-Belmonte, G.; Bisquert, J. Capacitance, spectroelectrochemistry and conductivity of polarons and bipolarons in a polydicarbazole based conducting polymer. J. Electroanal. Chem. 2008, 614, 49–60. [Google Scholar] [CrossRef]
- Vidanapathirana, K.P.; Careem, M.A.; Skaarup, S.; West, K. Ion movement in polypyrrole/dodecylbenzenesulphonate films in aqueous and non-aqueous electrolytes. Solid State Ionics 2002, 154–155, 331–335. [Google Scholar] [CrossRef]
- Tanaka, Y.; Hasegawa, T.; Shimamura, T.; Ukeda, H.; Ueda, T. Potentiometric evaluation of antioxidant capacity using polyoxometalate-immobilized electrodes. J. Electroanal. Chem. 2018, 828, 102–107. [Google Scholar] [CrossRef]
- Kosidlo, U.; Omastova, M.; Micusik, M.; Ciric-Marjanovic, G.; Randriamahazaka, H.; Wallmersperger, T.; Aabloo, A.; Kolaric, I.; Bauernhansl, T. Nanocarbon based ionic actuators-a review. Smart Mater. Struct. 2013, 22, 104022. [Google Scholar] [CrossRef]
- Baughman, R.H. Conducting polymer artificial muscles. Synth. Met. 1996, 78, 339–353. [Google Scholar] [CrossRef]
- Otero, T.F.; Boyano, I. Comparative study of conducting polymers by the ESCR model. J. Phys. Chem. B 2003, 107, 6730–6738. [Google Scholar] [CrossRef]
- Gao, Y.; Presser, V.; Zhang, L.; Niu, J.J.; McDonough, J.K.; Pérez, C.R.; Lin, H.; Fong, H.; Gogotsi, Y. High power supercapacitor electrodes based on flexible TiC-CDC nano-felts. J. Power Sources 2012, 201, 368–375. [Google Scholar] [CrossRef]
- Valero, L.; Otero, T.F.; Martinez, J.G.; Martínez, J.G. Exchanged Cations and Water during Reactions in Polypyrrole Macroions from Artificial Muscles. ChemPhysChem 2014, 15, 293–301. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, H.; Wu, X.; Wang, L.; Zhang, A.; Xia, T.; Dong, H.; Li, X.; Zhang, L. Progress of electrochemical capacitor electrode materials: A review. Int. J. Hydrogen Energy 2009, 34, 4889–4899. [Google Scholar] [CrossRef]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Gao, J.; Shang, K.; Ding, Y.; Wen, Z. Material and configuration design strategies towards flexible and wearable power supply devices: A review. J. Mater. Chem. A 2021, 9, 8950–8965. [Google Scholar] [CrossRef]
- Herrmann, S.; Aydemir, N.; Nägele, F.; Fantauzzi, D.; Jacob, T.; Travas-Sejdic, J.; Streb, C. Enhanced Capacitive Energy Storage in Polyoxometalate-Doped Polypyrrole. Adv. Funct. Mater. 2017, 27, 1–8. [Google Scholar] [CrossRef]
- Khuyen, N.Q.; Martinez, J.G.; Kaasik, F.; Tamm, T.; Otero, T.F.; Kiefer, R. Solvent effects on carbide-derived-carbon trilayer bending actuators. Synth. Met. 2019, 247, 170–176. [Google Scholar] [CrossRef]
- Kesküla, A.; Heinmaa, I.; Tamm, T.; Aydemir, N.; Travas-Sejdic, J.; Peikolainen, A.-L.; Kiefer, R. Improving the Electrochemical Performance and Stability of Polypyrrole by Polymerizing. Polymers 2020, 12, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genovese, M.; Lian, K. Polyoxometalate modified inorganic-organic nanocomposite materials for energy storage applications: A review. Curr. Opin. Solid State Mater. Sci. 2015, 19, 126–137. [Google Scholar] [CrossRef]
- Wu, C.; Wang, J.; Bai, Y.; Li, X. Significant effect of cations on polypyrrole cycle stability. Solid State Ionics 2020, 346, 115216. [Google Scholar] [CrossRef]








| PPy Films | Pristine σe (S cm−1) | σe (S cm−1) in NaClO4-PC | σe (S cm−1) in NaClO4-aq |
|---|---|---|---|
| PPyPT | 9.2 ± 0.65 | 6.3 ± 0.52 | 11.5 ± 1.06 |
| PPyPT-EG | 1.8 ± 0.12 | 0.7 ± 0.05 | 7.5 ± 0.64 |
| PPyCDC | 7.8 ± 0.68 | 4.8 ± 0.32 | 10.1 ± 0.84 |
| PPyCDC-EG | 1.5 ± 0.13 | 0.6 ± 0.04 | 6.6 ± 0.52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, C.B.; Zondaka, Z.; Le, Q.B.; Velmurugan, B.K.; Kiefer, R. Polypyrrole with Phosphor Tungsten Acid and Carbide-Derived Carbon: Change of Solvent in Electropolymerization and Linear Actuation. Materials 2021, 14, 6302. https://doi.org/10.3390/ma14216302
Tran CB, Zondaka Z, Le QB, Velmurugan BK, Kiefer R. Polypyrrole with Phosphor Tungsten Acid and Carbide-Derived Carbon: Change of Solvent in Electropolymerization and Linear Actuation. Materials. 2021; 14(21):6302. https://doi.org/10.3390/ma14216302
Chicago/Turabian StyleTran, Chau B., Zane Zondaka, Quoc Bao Le, Bharath Kumar Velmurugan, and Rudolf Kiefer. 2021. "Polypyrrole with Phosphor Tungsten Acid and Carbide-Derived Carbon: Change of Solvent in Electropolymerization and Linear Actuation" Materials 14, no. 21: 6302. https://doi.org/10.3390/ma14216302