Advanced Eco-Friendly Formulations of Guar Biopolymer-Based Textile Conditioners
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Sample Preparation
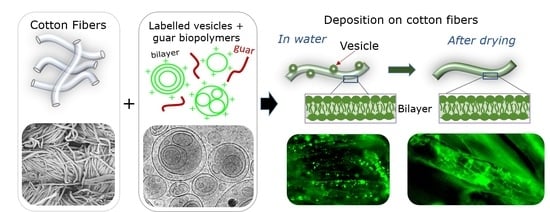
2.1.2. Deposition of Labeled TEQ Vesicles on Cotton Fabrics
2.2. Methods
3. Results and Discussion
3.1. Characterization of Vesicles Aqueous Dispersions
3.2. Characterization of Vesicles Dispersions in Presence of Guar Biopolymers
3.2.1. Microscopy
3.2.2. X-Ray Scattering
3.2.3. Rheology
3.3. Adsorption Studies on Cotton Fabrics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paderes, M.; Ahirwal, D.; Fernández Prieto, S. Natural and synthetic polymers in fabric and home care applications. Phys. Sci. Rev. 2017, 2, 203–234. [Google Scholar] [CrossRef]
- Pecquet, A.; McAvoy, D.; Pittinger, C.; Stanton, K. Polymers used in US household cleaning products: Assessment of data availability for ecological risk assessment. Integr. Environ. Assess. Manag. 2019, 15, 621–632. [Google Scholar] [CrossRef] [PubMed]
- DeLeo, P.C.; Summers, H.; Stanton, K.; Lam, M.W. Environmental risk assessment of polycarboxylate polymers used in cleaning products in the United States. Chemosphere 2020, 258, 127242. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.K.; Grandisson, C.; Golemanov, K.; Ahuja, R.; Berret, J.-F. Silicone incorporation into an esterquat based fabric softener in presence of guar polymers. Colloids Surf. A 2021, 615, 126175. [Google Scholar] [CrossRef]
- Hashem, M.; Ibrahim, N.A.; El-Shafei, A.; Refaie, R.; Hauser, P. An eco-friendly—Novel approach for attaining wrinkle—free/soft-hand cotton fabric. Carbohydr. Polym. 2009, 78, 690–703. [Google Scholar] [CrossRef]
- Mohamed, A.L.; Hassabo, A.G. Review of silicon-based materials for cellulosic fabrics with functional applications. J. Text. Color. Polym. Sci. 2019, 16, 139–157. [Google Scholar] [CrossRef]
- Crutzen, A.M. Study of the ditallowdimethylammonium chloride interaction with cellulose. J. Am. Oil Chem. Soc. 1995, 72, 137–143. [Google Scholar] [CrossRef]
- Murphy, D.S. Fabric softener technology: A review. J. Surfact. Deterg. 2015, 18, 199–204. [Google Scholar] [CrossRef]
- Kumar, A.; Trambitas, A.; Peggau, J.; Dahl, V.; Venzmer, J.; Gambaryan-Roisman, T.; Kleinen, J. Charge and size matters—How to formulate organomodified silicones for textile applications. Colloids Surf. A 2019, 560, 180–188. [Google Scholar] [CrossRef]
- Igarashi, T.; Nakamura, K.; Hoshi, M.; Hara, T.; Kojima, H.; Itou, M.; Ikeda, R.; Okamoto, Y. Elucidation of softening mechanism in rinse-cycle fabric softeners. part 2: Uneven adsorption—The key phenomenon to the effect of fabric softeners. J. Surfact. Deterg. 2016, 19, 759–773. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Gilson, L.; Henrich, F.; Dahl, V.; Kleinen, J.; Gambaryan-Roisman, T.; Venzmer, J. Intact deposition of cationic vesicles on anionic cellulose fibers: Role of vesicle size, polydispersity, and substrate roughness studied via streaming potential measurements. J. Colloid Interface Sci. 2016, 473, 152–161. [Google Scholar] [CrossRef]
- Kumar, A.; Dahl, V.; Kleinen, J.; Gambaryan-Roisman, T.; Venzmer, J. Influence of lipid bilayer phase behavior and substrate roughness on the pathways of intact vesicle deposition: A streaming potential study. Colloids Surf. A 2017, 521, 302–311. [Google Scholar] [CrossRef]
- Basavaraj, M.G.; McFarlane, N.L.; Lynch, M.L.; Wagner, N.J. Nanovesicle formation and microstructure in aqueous ditallowethylesterdimethylammonium chloride (DEEDMAC) solutions. J. Colloid Interface Sci. 2014, 429, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Chaudhuri, S.; Godfrin, M.P.; Mamak, M.; Reeder, B.; Hodgdon, T.; Saveyn, P.; Tripathi, A.; Bose, A. Impact of nearly water-insoluble additives on the properties of vesicular suspensions. Ind. Eng. Chem. Res. 2017, 56, 899–906. [Google Scholar] [CrossRef]
- Tekin, R.; Bac, N.; Erdogmus, H. Microencapsulation of fragrance and natural volatile oils for application in cosmetics, and household cleaning products. Macromol. Symp. 2013, 333, 35–40. [Google Scholar] [CrossRef]
- Mercadé-Prieto, R.; Pan, X.; Fernández-González, A.; Zhang, Z.; Bakalis, S. Quantification of microcapsules deposited in cotton fabrics before and after abrasion using fluorescence microscopy. Ind. Eng. Chem. Res. 2012, 51, 16741–16749. [Google Scholar] [CrossRef]
- Liu, D.; Yang, X.; Liu, P.; Mao, T.; Shang, X.; Wang, L. Synthesis and characterization of gemini ester surfactant and its application in efficient fabric softening. J. Mol. Liq. 2020, 299, 112236. [Google Scholar] [CrossRef]
- Aziz, H.A.; Abas, N.A.; Ping, B.T.Y.; Idris, Z. Transesterification of palm-based methyl palmitate into esteramine catalyzed by calcium oxide catalyst. J. Surfact. Deterg. 2020, 23, 251–262. [Google Scholar] [CrossRef]
- Beyer, R.M.; Durán, A.P.; Rademacher, T.T.; Martin, P.; Tayleur, C.; Brooks, S.E.; Coomes, D.; Donald, P.F.; Sanderson, F.J. The Environmental Impacts of Palm Oil and Its Alternatives . bioRxiv 2020. Available online: https://www.biorxiv.org/content/10.1101/2020.02.16.951301v1.full (accessed on 15 October 2015).
- Gaveau, D.L.; Sheil, D.; Husnayaen; Salim, M.A.; Arjasakusuma, S.; Ancrenaz, M.; Pacheco, P.; Meijaard, E. Rapid conversions and avoided deforestation: Examining four decades of industrial plantation expansion in Borneo. Sci. Rep. 2016, 6, 32017. [Google Scholar] [CrossRef]
- Ogorzałek, M.; Wasilewski, T.; Klimaszewska, E.; Zięba, M. Effect of hydrophobic plant extract on the physicochemical properties of a transparent fabric softener. J. Surfact. Deterg. 2020, 23, 405–413. [Google Scholar] [CrossRef]
- Pajnik, J.; Stamenić, M.; Radetić, M.; Tomanović, S.; Sukara, R.; Mihaljica, D.; Zizovic, I. Impregnation of cotton fabric with pyrethrum extract in supercritical carbon dioxide. J. Supercrit. Fluids 2017, 128, 66–72. [Google Scholar] [CrossRef]
- Artikboeva, R.; Yang, M.; Wu, Y.; Jie, C.; Heng, Q. Preparation and application of the hydrophilic amino-silicone softener by emulsion polymerization. Adv. Chem. Eng. Sci. 2020, 10, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Hsu, F.; Killinger, D.L.; Raders, S.M.; Sabelko, J.J.; Ming, Y.; Zhu, Y.; Brijmohan, S. Esterquat Free Liquid Fabric Softener Compositions Containing Unsaturated Fatty Acid Soap. International Patent Application No. PCT/US2018/057671, 5 February 2019. [Google Scholar]
- Zhang, H.Z.; Christov, N.; Jin, D.W. Composition Comprising a Quaternary Ammonium Compound, a Cationic Polysaccharide and a Nonionic Polysaccharide. International Patent Application No. WO 2015/192971 Al, 23 December 2015. [Google Scholar]
- Zhang, H.Z.; Jin, D.W.; Christov, N.; Cristobal, G. Compositions Comprising Quat and Polysaccharides. International Patent Application No. WO 2017/101798 Al, 22 June 2017. [Google Scholar]
- Johnson, W.; Heldreth, B.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; et al. Safety assessment of galactomannans as used in cosmetics. Int. J. Toxicol. 2015, 34, 35S–65S. [Google Scholar] [CrossRef] [PubMed]
- Hössel, P.; Dieing, R.; Nörenberg, R.; Pfau, A.; Sander, R. Conditioning polymers in today’s shampoo formulations—Efficacy, mechanism and test methods. J. Cosmetic Sci. 2000, 22, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ungewiß, J.; Vietzke, J.-P.; Rapp, C.; Schmidt-Lewerkühne, H.; Wittern, K.-P.; Salzer, R. Quantitative determination of cationic modified polysaccharides on hair using LC–MS and LC–MS–MS. Anal. Bioanal. Chem. 2005, 381, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Bujak, T.; Nizioł-Łukaszewska, Z.; Ziemlewska, A. Amphiphilic cationic polymers as effective substances improving the safety of use of body wash gels. Int. J. Bio. Macromol. 2020, 147, 973–979. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S.; Khatkar, B.S. Guar gum: Processing, properties and food applications—A review. J. Food Sci. Technol. 2014, 51, 409–418. [Google Scholar] [CrossRef] [Green Version]
- Khanal, A.; Cui, Y.; Zhang, L.; Pelton, R.; Ren, Y.; Ketelson, H.; Davis, J. Cationic liposome colloidal stability in the presence of guar derivatives suggests depletion interactions may be operative in artificial tears. Biomacromolecules 2010, 11, 2460–2464. [Google Scholar] [CrossRef]
- Gifford, P.; Evans, B.J.W.; Morris, J. A clinical evaluation of Systane. Cont. Lens Anterior Eye 2006, 29, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Jamshidian, M.; Savary, G.; Grisel, M.; Picard, C. Stretching properties of xanthan and hydroxypropyl guar in aqueous solutions and in cosmetic emulsions. Carbohydr. Polym. 2014, 112, 334–341. [Google Scholar] [CrossRef]
- Cheng, Y.; Brown, K.M.; Prud’homme, R.K. Characterization and intermolecular interactions of hydroxypropyl guar solutions. Biomacromolecules 2002, 3, 456–461. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Mousseau, F.; Christov, N.; Cristobal, G.; Vacher, A.; Airiau, M.; Bourgaux, C.; Heux, L.; Berret, J.-F. Fabric softener-cellulose nanocrystal interaction: A model for assessing surfactant deposition on cotton. J. Phys. Chem. B 2017, 121, 2299–2307. [Google Scholar] [CrossRef] [Green Version]
- Oikonomou, E.K.; Christov, N.; Cristobal, G.; Bourgaux, C.; Heux, L.; Boucenna, I.; Berret, J.-F. Design of eco-friendly fabric softeners: Structure, rheology and interaction with cellulose nanocrystals. J. Colloid Interface Sci. 2018, 525, 206–215. [Google Scholar] [CrossRef] [Green Version]
- Oikonomou, E.K.; Golemanov, K.; Dufils, P.-E.; Wilson, J.; Ahuja, R.; Heux, L.; Berret, J.-F. Cellulose nanocrystals mimicking micron-sized fibers to assess the deposition of latex particles on cotton. ACS Appl. Polym. Mat. 2021, 3, 3009–3018. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Messina, G.M.L.; Heux, L.; Marletta, G.; Berret, J.-F. Adsorption of a fabric conditioner on cellulose nanocrystals: Synergistic effects of surfactant vesicles and polysaccharides on softness properties. Cellulose 2021, 28, 2551–2566. [Google Scholar] [CrossRef]
- Mousseau, F.; Berret, J.-F.; Oikonomou, E.K. Design and applications of a fluorescent labeling technique for lipid and surfactant preformed vesicles. ACS Omega 2019, 4, 10485–10493. [Google Scholar] [CrossRef] [PubMed]
- Reimhult, E.; Höök, F.; Kasemo, B. Intact vesicle adsorption and supported biomembrane formation from vesicles in solution: Influence of surface chemistry, vesicle size, temperature, and osmotic pressure. Langmuir 2003, 19, 1681–1691. [Google Scholar] [CrossRef]
- Rubinstein, M.; Colby, R.H. Polymer Physics; Oxford University Press: Oxford, UK, 2010; p. 440. [Google Scholar]
- Saha, D.; Bhattacharya, S. Hydrocolloids as thickening and gelling agents in food: A critical review. J. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagyova, M.; Slovinska, L.; Blasko, J.; Grulova, I.; Kuricova, M.; Cigankova, V.; Harvanova, D.; Cizkova, D. A comparative study of PKH67, DiI, and BrdU labeling techniques for tracing rat mesenchymal stem cells. In Vitro Cell. Dev. Biol. Anim. 2014, 50, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Mousseau, F.; Vitorazi, L.; Herrmann, L.; Mornet, S.; Berret, J.-F. Polyelectrolyte assisted charge titration spectrometry: Applications to latex and oxide nanoparticles. J. Colloid Interface Sci. 2016, 475, 36–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Swaay, D.; DeMello, A. Microfluidic methods for forming liposomes. Lab. Chip 2013, 13, 752. [Google Scholar] [CrossRef] [PubMed]
- Mousseau, F.; Berret, J.-F. The role of surface charge in the interaction of nanoparticles with model pulmonary surfactants. Soft Matter 2018, 14, 5764–5774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousseau, F.; Oikonomou, E.K.; Vacher, A.; Airiau, M.; Mornet, S.; Berret, J.-F. Revealing the pulmonary surfactant corona on silica nanoparticles by cryo-transmission electron microscopy. Nanoscale Adv. 2020, 2, 642–647. [Google Scholar] [CrossRef] [Green Version]
- Seth, M.; Ramachandran, A.; Murch, B.P.; Leal, L.G. Origins of microstructural transformations in charged vesicle suspensions: The crowding hypothesis. Langmuir 2014, 30, 10176–10187. [Google Scholar] [CrossRef] [PubMed]
- Meyuhas, D.; Nir, S.; Lichtenberg, D. Aggregation of phospholipid vesicles by water-soluble polymers. Biophys. J. 1996, 71, 2602–2612. [Google Scholar] [CrossRef] [Green Version]
- Mousseau, F.; Le Borgne, R.; Seyrek, E.; Berret, J.-F. Biophysicochemical interaction of a clinical pulmonary surfactant with nanoalumina. Langmuir 2015, 31, 7346–7354. [Google Scholar] [CrossRef] [Green Version]
- Kawabata, Y.; Hayashi, K.; Kanao, T.; Ishikawa, A. Bilayer structure of ester-amide-type cationic surfactants in a dilute aqueous solution. Colloids Surf. A 2014, 441, 140–148. [Google Scholar] [CrossRef]
- Nagai, Y.; Kawabata, Y.; Kato, T. Microscopic investigation on morphologies of bilayer gel structure in the mixed polyoxyethylene-type nonionic surfactant systems. J. Phys. Chem. B 2012, 116, 12558–12566. [Google Scholar] [CrossRef]
- Kučerka, N.; Holland, B.W.; Gray, C.G.; Tomberli, B.; Katsaras, J. Scattering density profile model of POPG bilayers as determined by molecular dynamics simulations and small-angle neutron and X-ray scattering experiments. J. Phys. Chem. B 2012, 116, 232–239. [Google Scholar] [CrossRef] [Green Version]
- Chung, C.; Lee, M.; Choe, E. Characterization of cotton fabric scouring by FT-IR ATR spectroscopy. Carbohydr. Polym. 2004, 58, 417–420. [Google Scholar] [CrossRef]
- Obendorf, S.K.; Dixit, V.; Woo, D.J. Microscopy study of distribution of laundry fabric softener on cotton fabric. J. Surfact. Deterg. 2009, 12, 225–230. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oikonomou, E.K.; Berret, J.-F. Advanced Eco-Friendly Formulations of Guar Biopolymer-Based Textile Conditioners. Materials 2021, 14, 5749. https://doi.org/10.3390/ma14195749
Oikonomou EK, Berret J-F. Advanced Eco-Friendly Formulations of Guar Biopolymer-Based Textile Conditioners. Materials. 2021; 14(19):5749. https://doi.org/10.3390/ma14195749
Chicago/Turabian StyleOikonomou, Evdokia K., and Jean-François Berret. 2021. "Advanced Eco-Friendly Formulations of Guar Biopolymer-Based Textile Conditioners" Materials 14, no. 19: 5749. https://doi.org/10.3390/ma14195749