Optical Properties of Magnesium-Zinc Oxide for Thin Film Photovoltaics
Abstract
:1. Introduction
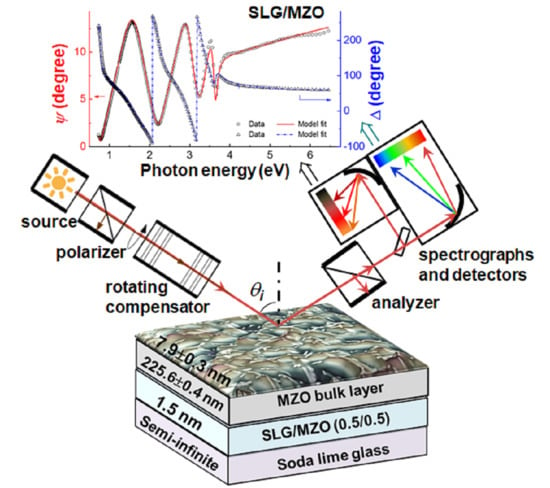
2. Materials and Methods
3. Results and Discussion
3.1. X-ray Diffraction (XRD)
3.2. Energy Dispersive X-ray Spectroscopy (EDS)
3.3. Parameterization of the MgxZn1−xO Complex Dielectric Function Spectra versus Mg Content x
3.4. Future Applications of Dielectric Function Parameterization for Characterization of CdTe Device Structures
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Disclaimer
References
- Green, M.; Dunlop, E.; Hohl-Ebinger, J.; Yoshita, M.; Kopidakis, N.; Hao, X. Solar cell efficiency tables (version 57). Prog. Photovolt. Res. Appl. 2021, 29, 3–15. [Google Scholar] [CrossRef]
- Gloeckler, M.; Sankin, I.; Zhao, Z. CdTe solar cells at the threshold to 20% efficiency. IEEE J. Photovolt. 2013, 3, 1389–1393. [Google Scholar] [CrossRef]
- Paudel, N.R.; Yan, Y. Enhancing the photo-currents of CdTe thin-film solar cells in both short and long wavelength regions. Appl. Phys. Lett. 2014, 105, 183510. [Google Scholar] [CrossRef]
- Geisthardt, R.M.; Topič, M.; Sites, J.R. Status and potential of CdTe solar-cell efficiency. IEEE J. Photovolt. 2015, 5, 1217–1221. [Google Scholar] [CrossRef]
- Sites, J.; Munshi, A.; Kephart, J.; Swanson, D.; Sampath, W.S. Progress and challenges with CdTe cell efficiency. In Proceedings of the 2016 IEEE 43rd Photovoltaic Specialists Conference (PVSC), Portland, OR, USA, 5–10 June 2016; IEEE: New York, NY, USA, 2016; pp. 3632–3635. [Google Scholar]
- Kanevce, A.; Reese, M.O.; Barnes, T.M.; Jensen, S.A.; Metzger, W.K. The roles of carrier concentration and interface, bulk, and grain-boundary recombination for 25% efficient CdTe solar cells. J. Appl. Phys. 2017, 121, 214506. [Google Scholar] [CrossRef]
- Metzger, W.K.; Grover, S.; Lu, D.; Colegrove, E.; Moseley, J.; Perkins, C.L.; Li, X.; Mallick, R.; Zhang, W.; Malik, R.; et al. Exceeding 20% efficiency with in situ group V doping in polycrystalline CdTe solar cell. Nat. Energy 2019, 4, 837–845. [Google Scholar] [CrossRef]
- Razooqi Alaani, M.A.; Koirala, P.; Pradhan, P.; Phillips, A.B.; Podraza, N.J.; Heben, M.J.; Collins, R.W. Tailoring the CdS/CdSe/CdTe multilayer structure for optimization of photovoltaic device performance guided by mapping spectroscopic ellipsometry. Sol. Energy Mater. Sol. Cells 2021, 221, 110907. [Google Scholar] [CrossRef]
- Colegrove, E.; Banai, R.; Blissett, C.; Buurma, C.; Ellsworth, J.; Morley, M.; Barnes, S.; Gilmore, C.; Bergeson, J.D.; Dhere, R.; et al. High-efficiency polycrystalline CdS/CdTe solar cells on buffered commercial TCO-coated glass. J. Electron. Mater. 2012, 41, 2833–2837. [Google Scholar] [CrossRef]
- Song, T.; Kanevce, A.; Sites, J.R. Emitter/absorber interface of CdTe solar cells. J. Appl. Phys. 2016, 119, 233104. [Google Scholar] [CrossRef]
- Ablekim, T.; Perkins, C.; Zheng, X.; Reich, C.; Swanson, D.; Colegrove, E.; Duenow, J.N.; Albin, D.; Nanayakkara, S.; Reese, M.O.; et al. Tailoring MgZnO/CdSeTe interfaces for photovoltaics. IEEE J. Photovolt. 2018, 9, 888–892. [Google Scholar] [CrossRef]
- Ren, S.; Li, H.; Lei, C.; Li, C.; Yin, X.; Wu, L.; Li, W.; Zhang, J.; Wang, W.; Feng, L. Interface modification to enhance electron extraction by deposition of a ZnMgO buffer on SnO2-coated FTO in CdTe solar cells. Sol. Energy 2019, 177, 545–552. [Google Scholar] [CrossRef]
- Awni, R.A.; Li, D.-B.; Song, Z.; Bista, S.S.; Razooqi, M.A.; Grice, C.R.; Chen, L.; Liyanage, G.K.; Li, C.; Phillips, A.B.; et al. Influences of buffer material and fabrication atmosphere on the electrical properties of CdTe solar cells. Prog. Photovolt. Res. Appl. 2019, 27, 1115–1123. [Google Scholar] [CrossRef]
- Kephart, J.M.; McCamy, J.W.; Ma, Z.; Ganjoo, A.; Alamgir, F.M.; Sampath, W.S. Band alignment of front contact layers for high-efficiency CdTe solar cells. Sol. Energy Mater. Sol. Cells 2016, 157, 266–275. [Google Scholar] [CrossRef] [Green Version]
- Li, D.-B.; Song, Z.; Awni, R.A.; Bista, S.S.; Shrestha, N.; Grice, C.R.; Chen, L.; Liyanage, G.K.; Razooqi, M.A.; Phillips, A.B.; et al. Eliminating s-kink to maximize the performance of MgZnO/CdTe solar cells. ACS Appl. Energy Mater. 2019, 2, 2896–2903. [Google Scholar] [CrossRef]
- Ohtomo, A.; Kawasaki, M.; Koida, T.; Masubuchi, K.; Koinuma, H. MgxZn1−xO as a II–VI widegap semiconductor alloy. Appl. Phys. Lett. 1998, 72, 2466–2468. [Google Scholar] [CrossRef] [Green Version]
- Minemoto, T.; Negami, T.; Nishiwaki, S.; Takakura, H.; Hamakawa, Y. Preparation of Zn1−xMgxO films by radio frequency magnetron sputtering. Thin Solid Films 2000, 372, 173–176. [Google Scholar] [CrossRef]
- Hsu, H.-C.; Wu, C.-Y.; Cheng, H.-M.; Hsieh, W.-F. Band gap engineering and stimulated emission of ZnMgO nanowires. Appl. Phys. Lett. 2006, 89, 013101. [Google Scholar] [CrossRef]
- Ferlito, E.P.; Ricciari, R.; Padalino, M.; Grasso, S.; Battaglia, A.; Sciuto, M.; Mello, D.; Tapfer, L.; Gerardi, C. Determination of Mg concentration and distribution in MgxZn1−xO films for photonic devices application. Surf. Interface Anal. 2014, 46, 823–826. [Google Scholar] [CrossRef]
- Amarasinghe, M.; Colegrove, E.; Moseley, J.; Moutinho, H.; Albin, D.; Duenow, J.; Jensen, S.; Kephart, J.; Sampath, W. Obtaining large columnar CdTe grains and long lifetime on nanocrystalline CdSe, MgZnO, or CdS layers. Adv. Energy Mater. 2018, 8, 1702666. [Google Scholar] [CrossRef]
- Rao, G.V.; Säuberlich, F.; Klein, A. Influence of Mg content on the band alignment at CdS/(Zn,Mg)O interfaces. Appl. Phys. Lett. 2005, 87, 032101. [Google Scholar] [CrossRef]
- Minemoto, T.; Hashimoto, Y.; Shams-Kolahi, W.; Satoh, T.; Negami, T.; Takakura, H.; Hamakawa, Y. Control of conduction band offset in wide-gap Cu(In,Ga)Se2 solar cells. Sol. Energy Mater. Sol. Cells 2014, 75, 121–126. [Google Scholar] [CrossRef]
- Ablekim, T.; Colegrove, E.; Metzger, W.K. Interface engineering for 25% CdTe solar cells. ACS Appl. Energy Mater. 2018, 1, 5135–5139. [Google Scholar] [CrossRef]
- Messier, R.; Giri, A.P.; Roy, R.A. Revised structure zone model for thin film physical structure. J. Vac. Sci. Technol. A 1984, 2, 500–503. [Google Scholar] [CrossRef]
- Mayes, E.L.H.; Murdoch, B.J.; Bilek, M.M.M.; McKenzie, D.R.; McCulloch, D.G.; Partridge, J.G. Co-deposition of band-gap tuned Zn1−xMgxO using high impulse power and dc-magnetron sputtering. J. Phys. D Appl. Phys. 2015, 48, 135301. [Google Scholar] [CrossRef]
- Coppa, B.J.; Davis, R.F.; Nemanich, R.J. Gold Schottky contacts on oxygen plasma-treated, n-type ZnO(0001). Appl. Phys. Lett. 2003, 82, 400–402. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Lee, C.-S.; Kim, S.; Chalapathy, R.B.V.; Al-Ammar, E.A.; Ahn, B.T. Understanding the light soaking effect of ZnMgO buffer in CIGS solar cells. Phys. Chem. Chem. Phys. (Inc. Faraday Trans.) 2015, 17, 19222–19229. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Wang, H.; Li, Y.; Li, H.; He, R.; Wu, L.; Li, W.; Zhang, J.; Wang, W.; Feng, L. Rapid thermal annealing on ZnMgO window layer for improved performance of CdTe solar cells. Sol. Energy Mater. Sol. Cells 2018, 187, 97–103. [Google Scholar] [CrossRef]
- Wang, A.; Tang, T.; Ren, S.; Zhang, J.; Wu, L.; Li, W.; Wang, W.; Feng, L. Characterization of co-sputtered MgxZn1−xO thin films and their application in CdTe solar cells. Mater. Sci. Semicond. Process. 2019, 94, 28–34. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Pan, X.; Wang, T.; Xie, E. The effects of thermal annealing on properties of MgxZn1−xO films by sputtering. J. Alloys Compd. 2009, 472, 208–210. [Google Scholar] [CrossRef]
- Kim, J.W.; Kang, H.S.; Kim, J.H.; Lee, S.Y. Variation of structural, electrical, and optical properties of Zn1−xMgxO thin films. J. Appl. Phys. 2006, 100, 033701. [Google Scholar] [CrossRef] [Green Version]
- Artegiani, E.; Leoncini, M.; Barbato, M.; Meneghini, M.; Meneghesso, G.; Cavallini, M.; Romeo, A. Analysis of magnesium zinc oxide layers for high efficiency CdTe devices. Thin Solid Films 2019, 672, 22–25. [Google Scholar] [CrossRef]
- Baines, T.; Durose, K.; Major, J.D. Co-sputtered MgxZn(1−x)O window layers for CdTe(1−x)Sex solar cells. In Proceedings of the 7th World Conference on Photovoltaic Energy Conversion (WCPEC) (A Joint Conference of 2018 IEEE 45th PVSC, 28th PVSEC & 34th EU PVSEC), Waikoloa, HI, USA, 10–15 June 2018; IEEE: New York, NY, USA, 2018; pp. 2974–2979. [Google Scholar]
- Bittau, F.; Potamialis, C.; Togay, M.; Abbas, A.; Isherwood, P.J.M.; Bowers, J.W.; Walls, J.M. Analysis and optimisation of the glass/TCO/MZO stack for thin film CdTe solar cells. Sol. Energy Mater. Sol. Cells 2018, 187, 15–22. [Google Scholar] [CrossRef]
- Bittau, F.; Artegiani, E.; Abbas, A.; Menossi, D.; Romeo, A.; Bowers, J.W.; Walls, J.M. Magnesium-doped zinc oxide as a high resistance transparent layer for thin film CdS/CdTe solar cells. In Proceedings of the 2017 IEEE 44th Photovoltaic Specialists Conference (PVSC), Washington, DC, USA, 25–30 June 2017; IEEE: New York, NY, USA, 2017; pp. 752–756. [Google Scholar]
- Ke, Y.; Berry, J.; Parilla, P.; Zakutayev, A.; O’Hayre, R.; Ginley, D. The origin of electrical property deterioration with increasing Mg concentration in ZnMgO:Ga. Thin Solid Films 2012, 520, 3697–3702. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, H.; Wang, Q.; Ma, J.; Zong, F.; Xiao, H.; Ji, F.; Hou, S. Structural and optical properties of MgxZn1−xO thin films deposited by magnetron sputtering. Phys. B Condens. Matter 2005, 364, 157–161. [Google Scholar] [CrossRef]
- Guan, W.L.; Lian, J.; Yu, Y.X.; Sun, Z.Z.; Zhao, M.L.; Wang, X.; Zhang, W.F. Optical properties of MgxZn1−xO thin films deposited on silicon and sapphire substrate by rf magnetron sputtering. Optik 2014, 125, 5167–5170. [Google Scholar] [CrossRef]
- Koster, R.S.; Fang, C.M.; Dijkstra, M.; van Blaaderen, A.; van Huis, M.A. Stabilization of rock salt ZnO nanocrystals by low-energy surfaces and Mg additions: A first-principles study. J. Phys. Chem. C 2015, 119, 5648–5656. [Google Scholar] [CrossRef]
- Kutlu-Narin, E.; Narin, P.; Yildiz, A.; Lisesivdin, S.B. Effect of magnesium content and growth temperature on structural and optical properties of USCVD-grown MgZnO films. Appl. Phys. A 2021, 127, 367. [Google Scholar] [CrossRef]
- Fan, X.F.; Sun, H.D.; Shen, Z.X.; Kuo, J.-L.; Lu, Y.M. A first-principle analysis on the phase stabilities, chemical bonds and band gaps of wurtzite structure AxZn1−xO alloys (A = Ca, Cd, Mg). J. Phys. Condens. Matter 2008, 20, 235221. [Google Scholar] [CrossRef] [PubMed]
- Choopun, S.; Vispute, R.D.; Yang, W.; Sharma, R.P.; Venkatesan, T. Realization of band gap above 5.0 eV in metastable cubic-phase MgxZn1−xO alloy films. Appl. Phys. Lett. 2002, 80, 1529–1531. [Google Scholar] [CrossRef]
- Maznichenko, I.V.; Ernst, A.; Bouhassoune, M.; Henk, J.; Däne, M.; Lüders, M.; Bruno, P.; Hergert, W.; Mertig, I.; Szotek, Z.; et al. Structural phase transitions and fundamental band gaps of MgxZn1−xO alloys from first principles. Phys. Rev. B 2009, 80, 144101. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.H.; Park, Y.R.; Kim, K.J. Spectroscopic ellipsometry study of Zn1−xMgxO thin films deposited on Al2O3(0001). Solid State Commun. 2000, 115, 127–130. [Google Scholar] [CrossRef]
- Cuong, H.B.; Le, N.M.; Jeong, S.-H.; Lee, B.-T. Tailoring of composition, band-gap, and structural phase in ZnMgO films by simply controlling growth temperature and oxygen partial pressure during sputter deposition. J. Alloys Compd. 2017, 709, 54–63. [Google Scholar] [CrossRef]
- Fujiwara, H.; Koh, J.; Rovira, P.I.; Collins, R.W. Assessment of effective-medium theories in the analysis of nucleation and microscopic surface roughness evolution for semiconductor thin films. Phys. Rev. B 2000, 61, 10832–10844. [Google Scholar] [CrossRef]
- Collins, R.W.; Vedam, K. Optical properties of solids. In Encyclopedia of Applied Physics; Trigg, G.L., Ed.; VCH: New York, NY, USA, 1995; Volume 12, pp. 285–336. [Google Scholar]
- Collins, R.W.; Ferlauto, A.S. Optical physics of materials. In Handbook of Ellipsometry; Tompkins, H.G., Irene, E.A., Eds.; William Andrew: Norwich, NY, USA, 2005; pp. 93–235. [Google Scholar]
- Koirala, P.; Li, J.; Podraza, N.J.; Collins, R.W. Real time and mapping spectroscopic ellipsometry for CdTe photovoltaics. In Spectroscopic Ellipsometry for Photovoltaics; Fundamental Principles and Solar Cell Characterization; Fujiwara, H., Collins, R.W., Eds.; Springer: Cham, Switzerland, 2018; Volume 1, pp. 357–412. [Google Scholar]
- Schmidt-Grund, R.; Schubert, M.; Rheinländer, B.; Fritsch, D.; Schmidt, H.; Kaidashev, E.M.; Lorenz, M.; Herzinger, C.M.; Grundmann, M. UV–VUV spectroscopic ellipsometry of ternary MgxZn1−xO (0 ≤ x ≤ 0.53) thin films. Thin Solid Films 2004, 455, 500–504. [Google Scholar] [CrossRef]
- Koirala, P.; Li, J.; Yoon, H.P.; Aryal, P.; Marsillac, S.; Rockett, A.A.; Podraza, N.J.; Collins, R.W. Through-the-glass spectroscopic ellipsometry for analysis of CdTe thin-film solar cells in the superstrate configuration. Prog. Photovolt. Res. Appl. 2016, 24, 1055–1067. [Google Scholar] [CrossRef]
- Niklasson, G.A.; Granqvist, C.G.; Hunderi, O. Effective medium models for the optical properties of inhomogeneous materials. Appl. Opt. 1981, 20, 26–30. [Google Scholar] [CrossRef]
- Koh, J.; Lu, Y.; Wronski, C.R.; Kuang, Y.; Collins, R.W.; Tsong, T.T.; Strausser, Y.E. Correlation of real time spectroellipsometry and atomic force microscopy measurements of surface roughness on amorphous semiconductor thin films. Appl. Phys. Lett. 1996, 69, 1297–1299. [Google Scholar] [CrossRef]
- Dahal, L.R.; Sainju, D.; Podraza, N.J.; Marsillac, S.; Collins, R.W. Real time spectroscopic ellipsometry of Ag/ZnO and Al/ZnO interfaces for back-reflectors in thin film Si:H photovoltaics. Thin Solid Films 2011, 519, 2682–2687. [Google Scholar] [CrossRef]
- Collins, R.W.; Koirala, P.; Podraza, N.J. Near-infrared to ultraviolet optical response and the absorption onset: Parametric representations. In The World Scientific Reference of Amorphous Materials; Structure, Properties, and Applications of Tetrahedrally Bonded Thin-Film Amorphous Semiconductors; Podraza, N.J., Collins, R.W., Eds.; World Scientific: Singapore, 2021; Volume 3, pp. 129–165. [Google Scholar]
- Oueslati, M.; Zouaghi, M.; Pistol, M.E.; Samuelson, L.; Grimmeiss, H.G.; Balkanski, M. Photoluminescence study of localization effects induced by the fluctuating random alloy potential in indirect band-gap GaAs1−xPx. Phys. Rev. B 1985, 32, 8220–8227. [Google Scholar] [CrossRef]
- Huso, J.; Che, H.; Thapa, D.; Canul, A.; McCluskey, M.D.; Bergman, L. Phonon dynamics and Urbach energy studies of MgZnO alloys. J. Appl. Phys. 2015, 117, 125702. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.; Sainju, D.; Wells, K.D.; Podraza, N.J.; Collins, R.W. Multilayer analysis of the CdTe solar cell structure by spectroscopic ellipsometry. In Proceedings of the 4th World Conference on Photovoltaic Energy Conversion (WCPEC), Waikoloa, HI, USA, 7–12 May 2006; IEEE: New York, NY, USA, 2006; pp. 475–478. [Google Scholar]
- Koirala, P.; Paudel, N.; Chen, J.; Attygalle, D.; Yan, Y.; Collins, R.W. Real time and post-deposition optical analysis of interfaces in CdTe solar cells. In Proceedings of the 2012 IEEE 38th Photovoltaic Specialists Conference, Austin, TX, USA, 3–8 June 2012; IEEE: New York, NY, USA, 2012; pp. 134–139. [Google Scholar]
- Koirala, P. Multichannel Spectroscopic Ellipsometry for CdTe Photovoltaics: From Materials and Interfaces to Solar Cells. Ph.D. Thesis, University of Toledo, Toledo, OH, USA, 2015. [Google Scholar]






| ZnO RF Power (Watt) | MgO RF Power (Watt) | Mg Content, x (Atomic Fraction) | MgxZn1−xO E0 = Eg (eV) |
|---|---|---|---|
| 240 | 0 | 0 | 3.264 ± 0.001 |
| 240 | 200 | 0.16 ± 0.02 | 3.433 ± 0.002 |
| 240 | 300 | 0.26 ± 0.05 | 3.554 ± 0.003 |
| 240 | 400 | 0.28 ± 0.07 | 3.613 ± 0.002 |
| 240 | 500 | 0.32 ± 0.06 | 3.690 ± 0.002 |
| 225 | 500 | 0.37 ± 0.03 | 3.781 ± 0.003 |
| 200 | 500 | 0.42 ± 0.07 | 3.826 ± 0.002 |
| Oscillator | Parameter | Value/Expression in Terms of Eg |
|---|---|---|
| E0 | A0 | 13.214 − 5.6414Eg + 0.879Eg2 (3.264 eV ≤ Eg ≤ 3.690 eV) 9700.7933 − 7900.0075Eg + 2145.2291Eg2 − 194.1559Eg3 (3.690 eV ≤ Eg ≤ 3.826 eV) |
| E0 (eV) | Eg | |
| Γ0 (eV) | −39.357 + 34.641Eg − 10.101Eg2 + 0.979Eg3 | |
| φ0 (degree) | −6.613 | |
| μ0 | 14.847 − 12.464Eg + 3.530Eg2 − 0.332Eg3 | |
| E1 | A1 | 3.902 |
| E1 (eV) | 8.754 | |
| Γ1 (eV) | 0.0248 | |
| φ1 (degree) | 113.156 | |
| μ1 | 0.392 | |
| ε1o | 1.729 | |
| Urbach absorption tail | Et (eV) | Eg |
| Eu (eV) | −32.548 + 28.609Eg − 8.3303Eg2 + 0.8054Eg3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alaani, M.A.R.; Koirala, P.; Phillips, A.B.; Liyanage, G.K.; Awni, R.A.; Sapkota, D.R.; Ramanujam, B.; Heben, M.J.; O’Leary, S.K.; Podraza, N.J.; et al. Optical Properties of Magnesium-Zinc Oxide for Thin Film Photovoltaics. Materials 2021, 14, 5649. https://doi.org/10.3390/ma14195649
Alaani MAR, Koirala P, Phillips AB, Liyanage GK, Awni RA, Sapkota DR, Ramanujam B, Heben MJ, O’Leary SK, Podraza NJ, et al. Optical Properties of Magnesium-Zinc Oxide for Thin Film Photovoltaics. Materials. 2021; 14(19):5649. https://doi.org/10.3390/ma14195649
Chicago/Turabian StyleAlaani, Mohammed A. Razooqi, Prakash Koirala, Adam B. Phillips, Geethika K. Liyanage, Rasha A. Awni, Dhurba R. Sapkota, Balaji Ramanujam, Michael J. Heben, Stephen K. O’Leary, Nikolas J. Podraza, and et al. 2021. "Optical Properties of Magnesium-Zinc Oxide for Thin Film Photovoltaics" Materials 14, no. 19: 5649. https://doi.org/10.3390/ma14195649