Structures, Bonding and Sensor Properties of Some Alkaline o-Phthalatocuprates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis of Alkaline di-o-Phthalatocuprates(II) Dihydrates
2.3. XRD Characterization
2.4. Vibrational Spectroscopy
2.5. Electrochemical Measurements
3. Results and Discussion
3.1. Crystal Structure
3.2. Vibrational Spectroscopy
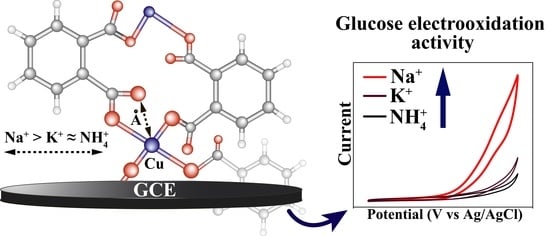
3.3. Sensor Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.-S. Metal—Organic frameworks for biosensing and bioimaging applications. Coord. Chem. Rev. 2017, 349, 139–155. [Google Scholar] [CrossRef]
- Li, J.; Xia, J.; Zhang, F.; Wang, Z.; Liu, Q. An electrochemical sensor based on copper-based metal-organic frameworks-graphene composites for determination of dihydroxybenzene isomers in water. Talanta 2018, 181, 80–86. [Google Scholar] [CrossRef]
- Anik, Ű.; Timur, S.; Dursun, Z. Metal organic frameworks in electrochemical and optical sensing platforms: A review. Microchim. Acta 2019, 186, 196. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Wei, Y.; Xing, T.; Cao, T.; Wu, S.; Zhu, F. A copper-based metal–organic framework/graphene nanocomposite for the sensitive and stable electrochemical detection of DNA bases. Analyst 2020, 145, 1933. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Li, X.; Qiu, Y.; Chen, Q.; Zhou, J.; Yang, Y.; Xie, Z.; Liu, P.; Cai, W.; Zhang, C. A simple modified electrode based on MIL-53(Fe) for the highly sensitive detection of hydrogen peroxide and nitrite. Anal. Methods 2017, 9, 2082–2088. [Google Scholar] [CrossRef]
- Brondani, D.; Zapp, E.; Heying, R.S.; Souza, B.; Vieira, I.C. Copper-based Metal-organic Framework Applied in the Development of an Electrochemical Biomimetic Sensor for Catechol Determination. Electroanalysis 2017, 29, 2810–2817. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, H.; Jiang, X.; Zhou, B. Electrochemical Determination of Aflatoxin B1 (AFB1) Using a Copper-Based Metal-Organic Framework (Cu-MOF) and Gold Nanoparticles (AuNPs) with Exonuclease III (Exo III) Assisted Recycling by Differential Pulse Voltammetry (DPV). Anal. Lett. 2019, 52, 2439–2453. [Google Scholar] [CrossRef]
- Song, Y.; Xu, M.; Gong, C.; Shen, Y.; Wang, L.; Xie, L.; Wang, L. Ratiometric electrochemical glucose biosensor based on GOD/AuNPs/Cu-BTC MOFs/macroporous carbon integrated electrode. Sens. Actuators B 2018, 257, 792–799. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, Y.; Yang, P.; Chen, Y.; Tao, J.; Hu, J.; Zhao, P. Carbon nanohorns enhanced electrochemical properties of Cu-based metal organic framework for ultrasensitive serum glucose sensing. J. Electroanalyt. Chem. 2020, 862, 114018. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Y.; Wang, N.; Xu, Q.; Xu, L.; Lin, M. Copper-based Metal-organic Framework for Non-enzymatic Electrochemical Detection of Glucose. Electroanalysis 2018, 30, 1011. [Google Scholar] [CrossRef] [Green Version]
- Cingi, M.B.; Lanfredi, A.M.M.; Tiripiccio, A.; Camellini, M.T. Influence of the alkaline cation on the structures of polymeric o-phthalatocuprate(II). II. The crystal structures of disodium di-o-phthalatocuprate(II) dihydrate and dipotassium catena-di-μ-(o-phthalato)-cuprate(II) dihydrate. Acta Cryst. 1978, B34, 412–416. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Cambridggge Crystallographic Data Centre, Accession Code 2101455. Available online: www.ccdc.cam.ac.uk/data_request/cif (accessed on 4 August 2021). by e/ailing data_request@ccdc.cam.ac.uk, or by con-tacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
- Mink, J.; Mink, L. Computer Program System for Vibrational Analyses of Polyatomic Molecules. (In Lahey-Fujitsu Fortran WIN32); Yumpu, Stockholm University: Stockholm, Sweden, 2004. [Google Scholar]
- Colombo, L.; Volovšek, V.; LePostollec, M. Vibrational Analysis and Normal Coordinate Calculations of o-Phthalic Acid Molecule. J. Raman Spectr. 1984, 15, 252–256. [Google Scholar] [CrossRef]
- Zhao, H.-X.; Zhuang, G.-L.; Wu, S.-T.; Long, L.-S.; Guo, H.-Y.; Ye, Z.-G.; Huang, R.-B.; Zheng, L.-S. Experimental and theoretical demonstration of ferroelectric anisotropy in a one-dimensional copper(II)-based coordination polymer. Chem. Comm. 2009, 1644–1646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.-L.; Sui, F.-F.; Lin, H.-Y.; Luan, J.; Liu, G.-C. Three Cu (II)/Co (II) Coordination Polymers Constructed From a Flexible Bis-pyridyl-bis-amide and Two Different Polycarboxylates: Assembly, Structure and Properties. Chin. J. Inorg. Chem. 2014, 30, 2626–2634. [Google Scholar] [CrossRef]
- Arenas, J.F.; Marcos, J.I. Infrared and Raman spectra of phthalate, isophthalate and terephthalate ions. Spectrochim. Acta 1979, 35A, 355–363. [Google Scholar] [CrossRef]
- Arenas, J.F.; Marcos, J.I. Infrared and Raman spectra of phthalic, isophthalic and terephthalic acids. Spectrochim. Acta 1980, 36A, 1075–1081. [Google Scholar] [CrossRef]
- De la Blanca, E.S.; Nũnez, J.L.; Martinez, P. Vibrational spectra of some o-substituted benzoic acid derivatives. J. Mol. Struct. 1986, 142, 45. [Google Scholar] [CrossRef]
- Loring, J.S.; Karlsson, M.; Fawcett, W.R.; Gasey, W.H. Infrared spectra of phthalic acid, the hydrogen phthalate ion, and the phthalate ion in aqueous solution. Spectrochim. Acta 2001, 57A, 1635–1642. [Google Scholar] [CrossRef]
- Tripathi, G.N.R.; Sheng, S.J. Solid-state vibrational spectra and structures of terephthalic acid and the terephthalate ion. J. Mol. Struct. 1979, 57, 21. [Google Scholar] [CrossRef]
- Geranmayeh, S.; Abbasi, A.; Skripkin, M.Y.; Badiei, A. A novel 2D zinc metal-organic framework: Synthesis, structural characterization and vibrational spectroscopic studies. Polyhedron 2012, 45, 204. [Google Scholar] [CrossRef]
- Geranmayeh, S.; Abbasi, A.; Zarnani, A.-H.; Skripkin, M.Y. A novel trinuclear zinc metal–organic network: Synthesis, X-ray diffraction structures, spectroscopic and biocompatibility studies. Polyhedron 2013, 61, 6. [Google Scholar] [CrossRef]
- Stepakova, L.V.; Skripkin, M.Y.; Chernykh, L.V.; Starova, G.L.; Hajba, L.; Mink, J.; Sandstrom, M. Vibrational spectroscopic and force field studies of copper(II) chloride and bromide compounds, and crystal structure of KcuBr. J. Raman Spectr. 2008, 39, 16. [Google Scholar] [CrossRef]
- Skripkin, M.Y.; Chernykh, L.V.; Pestova, O.N.; Baranauskaite, V.E.; Burkov, K.A.; Zamyatin, I.V.; Stepakova, L.V.; Gusev, I.M.; Gorbunov, A.O.; Bogachev, N.A.; et al. Influence of Interactions in Solutions on the Solid Phase Formation in Ternary Water-Salt Systems. Russ. J. General Chem. 2019, 89, 1085. [Google Scholar] [CrossRef]
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell. Mol. Neurobiol. 2019, 39, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef]
- Wu, L.; Lu, Z.; Ye, J. Biosensors and Bioelectronics Enzyme-Free Glucose Sensor Based on Layer-by-Layer Electrodeposition of Multilayer Films of Multi-Walled Carbon Nanotubes and Cu-Based Metal Framework Modified Glassy Carbon Electrode. Biosens. Bioelectron. 2019, 135, 45–49. [Google Scholar] [CrossRef]
- Shi, L.; Zhu, X.; Liu, T.; Zhao, H.; Lan, M. Sensors and Actuators B: Chemical Encapsulating Cu Nanoparticles into Metal-Organic Frameworks for Nonenzymatic Glucose Sensing. Sens. Actuators B Chem. 2016, 227, 583–590. [Google Scholar] [CrossRef]
- Pan, W.; Zheng, Z.; Wu, X.; Gao, J.; Liu, Y.; Yuan, Q.; Gan, W. Facile Synthesis of 2D/3D Hierarchical NiCu Bimetallic MOF for Non-Enzymatic Glucose Sensor. Microchem. J. 2021, 170, 106652. [Google Scholar] [CrossRef]
- Xue, Z.; Jia, L.; Zhu, R.; Du, L.; Zhao, Q. High-Performance Non-Enzymatic Glucose Electrochemical Sensor Constructed by Transition Nickel Modi Fi Ed Ni @ Cu-MOF. J. Electroanal. Chem. 2020, 858, 113783. [Google Scholar] [CrossRef]
- Hwang, D.-W.; Lee, S.; Seo, M.; Chung, T.D. Recent advances in electrochemical non-enzymatic glucose sensors: A review. Anal. Chim. Acta 2018, 1033, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Khairullina, E.M.; Panov, M.S.; Andriianov, V.S.; Ratautas, K.; Tumkin, I.I.; Răciukaitis, G. High rate fabrication of copper and copper–gold electrodes by laser-induced selective electroless plating for enzyme-free glucose sensing. RSC Adv. 2021, 11, 19521–19530. [Google Scholar] [CrossRef]







| Atom | Atom | Length/Å | Atom | Atom | Length/Å |
|---|---|---|---|---|---|
| Cu1 | O4 1 | 2.0060(8) | O1 | C1 | 1.2776(14) |
| Cu1 | O4 2 | 2.0060(8) | O3 | C8 | 1.2523(13) |
| Cu1 | O1 3 | 1.9474(8) | O2 | C1 | 1.2473(14) |
| Cu1 | O1 | 1.9474(8) | C1 | C2 | 1.5073(15) |
| O4 | Cu1 1 | 2.0060(8) | C8 | C7 | 1.4989(15) |
| O4 | C8 | 1.2758(13) |
| Contact | Distance, Å | ||
|---|---|---|---|
| I | II | III | |
| Cu-O1 | 1.969(4) | 1.999(5) | 2.0060(8) |
| Cu-O4 | 1.936(3) | 1.930(5) | 1.9474(8) |
| C1-O1 | 1.273(6) | 1.263(6) | 1.2776(14) |
| C1-O2 | 1.237(6) | 1.253(6) | 1.2473(14) |
| C8-O3 | 1.237(6) | 1.221(6) | 1.2523(13) |
| C8-O4 | 1.288(6) | 1.296(6) | 1.2758(13) |
| I | II | III | PED | Ass. | |||
|---|---|---|---|---|---|---|---|
| Exp. | Calc. | Exp. | Calc. | Exp. | Calc. | ||
| 1640 | 1639 | 1629 | 1629 | 1630 | 1630 | 75 ν(C=O), 24 ν(C-O) | νoop(C=O) |
| 1612 | 1613 | 1611 | 1611 | 1610 | 1611 | νip(C=O) | |
| 1263 | 1263 | 1270 | 1268 | 1259 | 1269 | 63 ν(C-O), 21 δ(CO2) | νoop(C-O) |
| 1265 | 1263 | 1258 | 1260 | 1252 | 1260 | νip(C-O) | |
| 660 | 660 | 661 | 660 | 658 | 657 | 68 δ(CO2), 13 ν(C-O) | δoop(CO2) |
| 653 | 654 | 651 | 652 | 653 | 653 | δip(CO2) | |
| 317 | 317 | 319 | 319 | 318 | 317 | 70 ν(CuO), 30 δ(CuOC) | νa(CuO) |
| 305 | 305 | 307 | 307 | 306 | 306 | νs(CuO) | |
| 277 | 275 | 272 | 273 | 271 | 269 | 90 τ(CO) | τoop(CO) |
| 267 | 268 | 260 | 261 | 260 | 260 | τip(CO) | |
| Force Constant | I | II | III | Acid [16] |
|---|---|---|---|---|
| Stretch | ||||
| Cu-O | 1.465 | 1.460 | 1.462 | |
| C-O | 6.022 | 6.071 | 6.057 | 5.93 |
| C=O | 8.409 | 8.621 | 8.616 | 8.5 |
| C-C | 2.503 | 2.541 | 2.539 | 2.6 |
| Stretch-stretch | ||||
| Cu-O, Cu-O (trans) | −0.132 | −0.167 | −0.175 | |
| C-O, Cu-O | 0.116 | 0.125 | 0.112 | |
| Bending | ||||
| CO2 | 1.654 | 1.578 | 1.589 | 1.65 |
| CO—torsion | 0.266 | 0.277 | 0.263 | 0.21 |
| Electrode Material | Linear Range (µM) | LOD (µM) | Refs. |
|---|---|---|---|
| Cu-MOF/CNHs/GCE | 0.25–1200 | 0.078 | 30 |
| Multiplayer films of Cu-MOF/MWNTs | 0.5–2340 | 0.4 | 9 |
| Cu-in-ZIF-8 | Up to 700 | 2.76 | 31 |
| NiCu-MOF-6 | 20–4930 | 15 | 32 |
| Ni@Cu-MOF | 5–2500 | 1.67 | 33 |
| Cu-MOF | 10–3500 | 2.4 | 10 |
| Sodium o-phthalatocuprate/GCE | 1–3000 | 0.26 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gladnev, S.V.; Grigoryev, M.V.; Kryukova, M.A.; Khairullina, E.M.; Tumkin, I.I.; Bogachev, N.A.; Mereshchenko, A.S.; Skripkin, M.Y. Structures, Bonding and Sensor Properties of Some Alkaline o-Phthalatocuprates. Materials 2021, 14, 5548. https://doi.org/10.3390/ma14195548
Gladnev SV, Grigoryev MV, Kryukova MA, Khairullina EM, Tumkin II, Bogachev NA, Mereshchenko AS, Skripkin MY. Structures, Bonding and Sensor Properties of Some Alkaline o-Phthalatocuprates. Materials. 2021; 14(19):5548. https://doi.org/10.3390/ma14195548
Chicago/Turabian StyleGladnev, Sergey V., Mikhail V. Grigoryev, Mariya A. Kryukova, Evgenia M. Khairullina, Ilya I. Tumkin, Nikita A. Bogachev, Andrey S. Mereshchenko, and Mikhail Y. Skripkin. 2021. "Structures, Bonding and Sensor Properties of Some Alkaline o-Phthalatocuprates" Materials 14, no. 19: 5548. https://doi.org/10.3390/ma14195548