Multinucleated Giant Cells Induced by a Silk Fibroin Construct Express Proinflammatory Agents: An Immunohistological Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Three-Dimensional Nonwoven Silk Fibroin Scaffolds
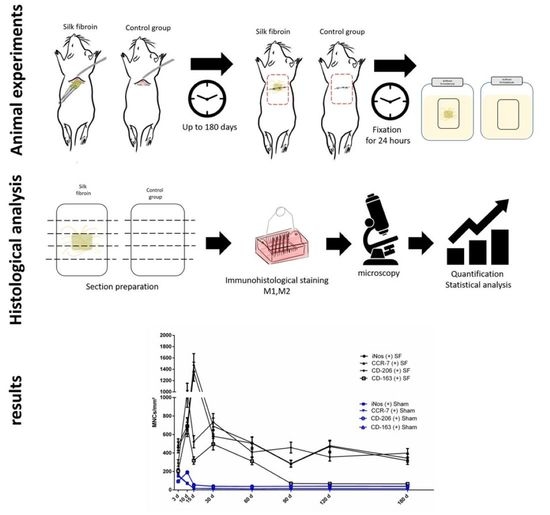
2.2. Animal Experiments
2.3. Histological and Immunohistological Staining
2.4. Histomorphometric Analysis
2.5. Statistical Analysis
3. Results
3.1. Qualitative Analysis
3.1.1. Control Group
3.1.2. Silk Fibroin Group
3.2. Quantitative Analysis
3.2.1. Differentiation of Macrophages over the Evaluation Time Points
3.2.2. Kinetics of Macrophage Polarization over the Evaluation Time Period
3.2.3. Differentiation of Multinucleated Giant Cells over the Evaluation Time Period
3.2.4. Kinetics of Multinucleated Giant Cell Polarization over the Evaluation Time Period
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vepari, C.; Kaplan, D.L. Silk as a biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef] [PubMed]
- Bottino, M.C.; Thomas, V.; Schmidt, G.; Vohra, Y.K.; Chu, T.-M.G.; Kowolik, M.J.; Janowski, G. Recent advances in the development of GTR/GBR membranes for periodontal regeneration—A materials perspective. Dent. Mater. 2012, 28, 703–721. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chia-Lai, P.-J.; Orlowska, A.; Al-Maawi, S.; Dias, A.; Zhang, Y.; Wang, X.; Zender, N.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Sugar-based collagen membrane cross-linking increases barrier capacity of membranes. Clin. Oral Investig. 2017, 22, 1851–1863. [Google Scholar] [CrossRef] [PubMed]
- Greene, A.H.; Bumgardner, J.D.; Yang, Y.; Moseley, J.; Haggard, W.O. Chitosan-coated Stainless Steel Screws for Fixation in Contaminated Fractures. Clin. Orthop. Relat. Res. 2008, 466, 1699–1704. [Google Scholar] [CrossRef] [Green Version]
- Boateng, J.; Matthews, K.; Stevens, H.N.; Eccleston, G.M. Wound Healing Dressings and Drug Delivery Systems: A Review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef] [PubMed]
- Montoya, N.V.; Peterson, R.; Ornell, K.J.; Albrecht, D.R.; Coburn, J.M. Silk Particle Production Based on Silk/PVA Phase Separation Using a Microfabricated Co-flow Device. Molecules 2020, 25, 890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ornell, K.J.; Taylor, J.S.; Zeki, J.; Ikegaki, N.; Shimada, H.; Coburn, J.M.; Chiu, B. Local delivery of dinutuximab from lyophilized silk fibroin foams for treatment of an orthotopic neuroblastoma model. Cancer Med. 2020, 9, 2891–2903. [Google Scholar] [CrossRef] [Green Version]
- Sanz-Fraile, H.; Amoros, S.; Mendizabal, M.I.I.; Galvez-Monton, C.; Prat-Vidal, C.; Bayes-Genis, A.; Navajas, D.; Farre, R.; Otero, J. Silk-Reinforced Collagen Hydrogels with Raised Multiscale Stiffness for Mesenchymal Cells 3D Culture. Tissue Eng. Part A 2020, 26, 358–370. [Google Scholar] [CrossRef]
- Tanaka, K.; Fukuda, D.; Higashikuni, Y.; Hirata, Y.; Komuro, I.; Saotome, T.; Yamashita, Y.; Asakura, T.; Sata, M. Biodegradable Extremely-Small-Diameter Vascular Graft Made of Silk Fibroin can be Implanted in Mice. J. Atheroscler. Thromb. 2020, 27, 1299–1309. [Google Scholar] [CrossRef] [Green Version]
- Hadisi, Z.; Farokhi, M.; Bakhsheshi-Rad, H.R.; Jahanshahi, M.; Hasanpour, S.; Pagan, E.; Dolatshahi-Pirouz, A.; Zhang, Y.S.; Kundu, S.C.; Akbari, M. Hyaluronic Acid (HA)-Based Silk Fibroin/Zinc Oxide Core–Shell Electrospun Dressing for Burn Wound Management. Macromol. Biosci. 2020, 20, e1900328. [Google Scholar] [CrossRef] [PubMed]
- Al-Maawi, S.; Orlowska, A.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. In vivo cellular reactions to different biomaterials—Physiological and pathological aspects and their consequences. Semin. Immunol. 2017, 29, 49–61. [Google Scholar] [CrossRef]
- Figueiredo, M.; Henriques, J.; Martins, G.; Guerra, F.; Judas, F.; Figueiredo, H. Physicochemical characterization of biomaterials commonly used in dentistry as bone substitutes-Comparison with human bone. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 92, 409–419. [Google Scholar] [CrossRef]
- Lorenz, J.; Korzinskas, T.; Chia, P.; Al Maawi, S.; Eichler, K.; Sader, R.A.; Ghanaati, S. Do Clinical and Radiological Assessments Contribute to the Understanding of Biomaterials? Results from a Prospective Randomized Sinus Augmentation Split-Mouth Trial. J. Oral Implant. 2018, 44, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Goissis, G.; Marcantonio, E.; Marcantonio, R.A.; Lia, R.C.C.; Cancian, D.C.; De Carvalho, W.M. Biocompatibility studies of anionic collagen membranes with different degree of glutaraldehyde cross-linking. Biomater. 1999, 20, 27–34. [Google Scholar] [CrossRef]
- Ghanaati, S.; Schlee, M.; Webber, M.; Willershausen, I.; Barbeck, M.; Balic, E.; Görlach, C.; I Stupp, S.; A Sader, R.; Kirkpatrick, C.J. Evaluation of the tissue reaction to a new bilayered collagen matrix in vivo and its translation to the clinic. Biomed. Mater. 2011, 6, 015010. [Google Scholar] [CrossRef]
- Brodbeck, W.G.; MacEwan, M.; Colton, E.; Meyerson, H.; Anderson, J.M. Lymphocytes and the foreign body response: Lymphocyte enhancement of macrophage adhesion and fusion. J. Biomed. Mater. Res. Part A 2005, 74, 222–229. [Google Scholar] [CrossRef]
- Brown, B.N.; Valentin, J.E.; Stewart-Akers, A.M.; McCabe, G.P.; Badylak, S.F. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomater. 2009, 30, 1482–1491. [Google Scholar] [CrossRef] [Green Version]
- Mills, C.D.; Ley, K. M1 and M2 Macrophages: The Chicken and the Egg of Immunity. J. Innate Immun. 2014, 6, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Lisi, L.; Ciotti, G.; Braun, D.; Kalinin, S.; Currò, D.; Russo, C.D.; Coli, A.; Mangiola, A.; Anile, C.; Feinstein, D.; et al. Expression of iNOS, CD163 and ARG-1 taken as M1 and M2 markers of microglial polarization in human glioblastoma and the surrounding normal parenchyma. Neurosci. Lett. 2017, 645, 106–112. [Google Scholar] [CrossRef]
- Mariani, E.; Lisignoli, G.; Borzì, R.M.; Pulsatelli, L. Biomaterials: Foreign Bodies or Tuners for the Immune Response? Int. J. Mol. Sci. 2019, 20, 636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, A.; Sinha, M.; Datta, S.; Abas, M.; Chaffee, S.; Sen, C.K.; Roy, S. Monocyte and Macrophage Plasticity in Tissue Repair and Regeneration. Am. J. Pathol. 2015, 185, 2596–2606. [Google Scholar] [CrossRef] [Green Version]
- Miron, R.J.; Bosshardt, D.D. Multinucleated Giant Cells: Good Guys or Bad Guys? Tissue Eng. Part B Rev. 2018, 24, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Brodbeck, W.G.; Anderson, J.M. Giant cell formation and function. Curr. Opin. Hematol. 2009, 16, 53–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Maawi, S.; Rutkowski, J.L.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. The Biomaterial-Induced Cellular Reaction Allows a Novel Classification System Regardless of the Biomaterials Origin. J. Oral Implant. 2020, 46, 190–207. [Google Scholar] [CrossRef]
- Barbeck, M.; Dard, M.; Kokkinopoulou, M.; Markl, J.; Booms, P.; A Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Small-sized granules of biphasic bone substitutes support fast implant bed vascularization. Biomatter 2015, 5, e1056943. [Google Scholar] [CrossRef] [Green Version]
- Ghanaati, S.; Barbeck, M.; Orth, C.; Willershausen, I.; Thimm, B.W.; Hoffmann, C.; Rasic, A.; Sader, R.A.; Unger, R.E.; Peters, F. Influence of β-tricalcium phosphate granule size and morphology on tissue reaction in vivo. Acta Biomater. 2010, 6, 4476–4487. [Google Scholar] [CrossRef]
- Ghanaati, S.; Orth, C.; Unger, R.E.; Barbeck, M.; Webber, M.J.; Motta, A.; Migliaresi, C.; Kirkpatrick, C.J. Fine-tuning scaffolds for tissue regeneration: Effects of formic acid processing on tissue reaction to silk fibroin. J. Tissue Eng. Regen. Med. 2010, 4, 464–472. [Google Scholar] [CrossRef]
- Ghanaati, S. Non-cross-linked porcine-based collagen I–III membranes do not require high vascularization rates for their integration within the implantation bed: A paradigm shift. Acta Biomater. 2012, 8, 3061–3072. [Google Scholar] [CrossRef]
- Armato, U.; Dal Pra, I.; Migliaresi, C.; Motta, A.; Kesenci, K. Method for the Preparation of a Non-Woven Silk Fibroin Fabrics. Patent nr. US20040097709A1, 2007. [Google Scholar]
- Zhang, Y.; Al-Maawi, S.; Wang, X.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Biomaterial-induced multinucleated giant cells express proinflammatory signaling molecules: A histological study in humans. J. Biomed. Mater. Res. Part A 2018, 107, 780–790. [Google Scholar] [CrossRef]
- Barbeck, M.; Motta, A.; Migliaresi, C.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Heterogeneity of biomaterial-induced multinucleated giant cells: Possible importance for the regeneration process? J. Biomed. Mater. Res. Part A 2015, 104, 413–418. [Google Scholar] [CrossRef]
- Franz, S.; Allenstein, F.; Kajahn, J.; Forstreuter, I.; Hintze, V.; Möller, S.; Simon, J.C. Artificial extracellular matrices composed of collagen I and high-sulfated hyaluronan promote phenotypic and functional modulation of human pro-inflammatory M1 macrophages. Acta Biomater. 2013, 9, 5621–5629. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, J.; Kubesch, A.; Al-Maawi, S.; Schwarz, F.; Sader, R.A.; Schlee, M.; Ghanaati, S. Allogeneic bone block for challenging augmentation—A clinical, histological, and histomorphometrical investigation of tissue reaction and new bone formation. Clin. Oral Investig. 2018, 22, 3159–3169. [Google Scholar] [CrossRef] [PubMed]
- Brodbeck, W.G.; Patel, J.; Voskerician, G.; Christenson, E.; Shive, M.S.; Nakayama, Y.; Matsuda, T.; Ziats, N.P.; Anderson, J.M. Biomaterial adherent macrophage apoptosis is increased by hydrophilic and anionic substrates in vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 10287–10292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghanaati, S.; Barbeck, M.; Detsch, R.; Deisinger, U.; Hilbig, U.; Rausch, V.; Sader, R.; E Unger, R.; Ziegler, G.; Kirkpatrick, C.J. The chemical composition of synthetic bone substitutes influences tissue reactions in vivo: Histological and histomorphometrical analysis of the cellular inflammatory response to hydroxyapatite, beta-tricalcium phosphate and biphasic calcium phosphate ceramics. Biomed. Mater. 2012, 7, 015005. [Google Scholar] [CrossRef]
- Klopfleisch, R.; Jung, F. The pathology of the foreign body reaction against biomaterials. J. Biomed. Mater. Res. Part A 2017, 105, 927–940. [Google Scholar] [CrossRef] [PubMed]
- Al-Maawi, S.; Herrera-Vizcaíno, C.; Orlowska, A.; Willershausen, I.; Sader, R.; Miron, R.J.; Choukroun, J.; Ghanaati, S. Biologization of Collagen-Based Biomaterials Using Liquid-Platelet-Rich Fibrin: New Insights into Clinically Applicable Tissue Engineering. Materials 2019, 12, 3993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klopfleisch, R. Macrophage reaction against biomaterials in the mouse model – Phenotypes, functions and markers. Acta Biomater. 2016, 43, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nat. Cell Biol. 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Jones, J.A.; Dadsetan, M.; Collier, T.O.; Ebert, M.; Stokes, K.S.; Ward, R.S.; Hiltner, P.A.; Anderson, J.M. Macrophage behavior on surface-modified polyurethanes. J. Biomater. Sci. Polym. Ed. 2004, 15, 567–584. [Google Scholar] [CrossRef] [PubMed]
- Brodbeck, W.G.; Shive, M.S.; Colton, E.; Nakayama, Y.; Matsuda, T.; Anderson, J.M. Influence of biomaterial surface chemistry on the apoptosis of adherent cells. J. Biomed. Mater. Res. 2001, 55, 661–668. [Google Scholar] [CrossRef]
- Christo, S.N.; Diener, K.; Bachhuka, A.; Vasilev, K.; Hayball, J.D. Innate Immunity and Biomaterials at the Nexus: Friends or Foes. BioMed Res. Int. 2015, 2015, 342304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrera-Vizcaino, C.; Al-Maawi, S.; Sader, R.; Kirkpatrick, C.J.; Choukroun, J.; Ghanaati, S. Modification of collagen-based sponges can induce an upshift of the early inflammatory response and a chronic inflammatory reaction led by M1 macrophages: An in vivo study. Clin. Oral Investig. 2020, 24, 3485–3500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Maawi, S.; Vorakulpipat, C.; Orlowska, A.; Zrnc, T.A.; Sader, R.A.; Kirkpatrick, C.J.; Ghanaati, S. In vivo Implantation of a Bovine-Derived Collagen Membrane Leads to Changes in the Physiological Cellular Pattern of Wound Healing by the Induction of Multinucleated Giant Cells: An Adverse Reaction? Front. Bioeng. Biotechnol. 2018, 6, 104. [Google Scholar] [CrossRef] [PubMed]
- McNally, A.K.; Anderson, J.M. Foreign body-type multinucleated giant cells induced by interleukin-4 express select lymphocyte co-stimulatory molecules and are phenotypically distinct from osteoclasts and dendritic cells. Exp. Mol. Pathol. 2011, 91, 673–681. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.M.; Jones, J.A. Phenotypic dichotomies in the foreign body reaction. Biomater. 2007, 28, 5114–5120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNally, A.K.; MacEwan, S.R.; Anderson, J.M. Foreign body-type multinucleated giant cell formation requires protein kinase C β, δ, and ζ. Exp. Mol. Pathol. 2008, 84, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Underhill, D.M.; Goodridge, H.S. Information processing during phagocytosis. Nat. Rev. Immunol. 2012, 12, 492–502. [Google Scholar] [CrossRef] [Green Version]
- Rosas, M.; Liddiard, K.; Kimberg, M.; Faro-Trindade, I.; McDonald, J.U.; Williams, D.L.; Brown, G.; Taylor, P. The Induction of Inflammation by Dectin-1 In Vivo Is Dependent on Myeloid Cell Programming and the Progression of Phagocytosis. J. Immunol. 2008, 181, 3549–3557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milde, R.; Ritter, J.; Tennent, G.A.; Loesch, A.; Martinez, F.O.; Gordon, S.; Pepys, M.B.; Verschoor, A.; Helming, L. Multinucleated Giant Cells Are Specialized for Complement-Mediated Phagocytosis and Large Target Destruction. Cell Rep. 2015, 13, 1937–1948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbeck, M.; Booms, P.; Unger, R.; Hoffmann, V.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Multinucleated giant cells in the implant bed of bone substitutes are foreign body giant cells-New insights into the material-mediated healing process. J. Biomed. Mater. Res. Part A 2017, 105, 1105–1111. [Google Scholar] [CrossRef]
- Kyriakides, T.R.; Foster, M.J.; Keeney, G.E.; Tsai, A.; Giachelli, C.M.; Clark-Lewis, I.; Rollins, B.J.; Bornstein, P. The CC Chemokine Ligand, CCL2/MCP1, Participates in Macrophage Fusion and Foreign Body Giant Cell Formation. Am. J. Pathol. 2004, 165, 2157–2166. [Google Scholar] [CrossRef] [Green Version]
- Khan, U.A.; Hashimi, S.M.; Khan, S.; Quan, J.; Bakr, M.; Forwood, M.R.; Morrison, N.M. Differential Expression of Chemokines, Chemokine Receptors and Proteinases by Foreign Body Giant Cells (FBGCs) and Osteoclasts. J. Cell. Biochem. 2014, 115, 1290–1298. [Google Scholar] [CrossRef] [Green Version]
- Kajahn, J.; Franz, S.; Rueckert, E.; Forstreuter, I.; Hintze, V.; Moeller, S.; Simon, J.C. Artificial extracellular matrices composed of collagen I and high sulfated hyaluronan modulate monocyte to macrophage differentiation under conditions of sterile inflammation. Biomatter 2012, 2, 226–273. [Google Scholar] [CrossRef] [Green Version]
- Lucke, S.; Walschus, U.; Hoene, A.; Schnabelrauch, M.; Nebe, J.B.; Finke, B.; Schlosser, M. The in vivo inflammatory and foreign body giant cell response against different poly(l -lactide-co- d/l -lactide) implants is primarily determined by material morphology rather than surface chemistry. J. Biomed. Mater. Res. Part A 2018, 106, 2726–2734. [Google Scholar] [CrossRef] [PubMed]
- Dadsetan, M.; Jones, J.A.; Hiltner, A.; Anderson, J.M. Surface chemistry mediates adhesive structure, cytoskeletal organization, and fusion of macrophages. J. Biomed. Mater. Res. 2004, 71, 439–448. [Google Scholar] [CrossRef]
- McNally, A.K.; Anderson, J.M. Multinucleated giant cell formation exhibits features of phagocytosis with participation of the endoplasmic reticulum. Exp. Mol. Pathol. 2005, 79, 126–135. [Google Scholar] [CrossRef]
- Lorenz, J.; Kubesch, A.; Korzinskas, T.; Barbeck, M.; Landes, C.; Sader, R.A.; Kirkpatrick, C.J.; Ghanaati, S. TRAP-Positive Multinucleated Giant Cells Are Foreign Body Giant Cells Rather Than Osteoclasts: Results From a Split-Mouth Study in Humans. J. Oral Implant. 2015, 41, e257–e266. [Google Scholar] [CrossRef] [Green Version]
- Ghanaati, S.; Barbeck, M.; Lorenz, J.; Stuebinger, S.; Seitz, O.; Landes, C.; Kovács, A.F.; Kirkpatrick, C.J.; Sader, R. Synthetic bone substitute material comparable with xenogeneic material for bone tissue regeneration in oral cancer patients: First and preliminary histological, histomorphometrical and clinical results. Ann. Maxillofac. Surg. 2013, 3, 126–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorenz, J.; Barbeck, M.; Sader, R.A.; Kirkpatrick, C.J.; Russe, P.; Choukroun, J.; Ghanaati, S. Foreign Body Giant Cell–Related Encapsulation of a Synthetic Material Three Years After Augmentation. J. Oral Implant. 2016, 42, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.; Walters, L.M. Tartrate-resistant acid phosphatase in bone and cartilage following decalcification and cold-embedding in plastic. J. Histochem. Cytochem. 1987, 35, 203–206. [Google Scholar] [CrossRef] [Green Version]
- Ghanaati, S.; Udeabor, S.E.; Barbeck, M.; Willershausen, I.; Kuenzel, O.; Sader, R.A.; Kirkpatrick, C.J. Implantation of silicon dioxide-based nanocrystalline hydroxyapatite and pure phase beta-tricalciumphosphate bone substitute granules in caprine muscle tissue does not induce new bone formation. Head Face Med. 2013, 9, 1. [Google Scholar] [CrossRef] [Green Version]
- Ghanaati, S.; Orth, C.; Barbeck, M.; Willershausen, I.; Thimm, B.W.; Booms, P.; Stübinger, S.; Landes, C.; Sader, R.A.; Kirkpatrick, C.J. Histological and histomorphometrical analysis of a silica matrix embedded nanocrystalline hydroxyapatite bone substitute using the subcutaneous implantation model in Wistar rats. Biomed. Mater. 2010, 5, 035005. [Google Scholar] [CrossRef]
- Janckila, A.J.; Slone, S.P.; Lear, S.C.; Martin, A.; Yam, L.T. Tartrate-Resistant Acid Phosphatase as an Immunohistochemical Marker for Inflammatory Macrophages. Am. J. Clin. Pathol. 2007, 127, 556–566. [Google Scholar] [CrossRef]
- Barbeck, M.; Lorenz, J.; Holthaus, M.G.; Raetscho, N.; Kubesch, A.; Booms, P.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Porcine Dermis and Pericardium-Based, Non–Cross-Linked Materials Induce Multinucleated Giant Cells After Their In Vivo Implantation: A Physiological Reaction? J. Oral Implant. 2015, 41, e267–e281. [Google Scholar] [CrossRef]








| Antibody | Dilution | Pretreatment |
|---|---|---|
| CD-68 (MCA341GA, Bio-Rad, CA, USA) | 1:600 | Citrate buffer(pH = 6.0) |
| iNOS (ab15323, Abcam, UK) | 1:1000 | Citrate buffer(pH = 6.0) |
| CCR-7 (ab32527, Abcam, UK) | 1:2000 | Citrate buffer(pH = 6.0) |
| CD-206 (ab64693, Abcam, UK) | 1:2000 | Citrate buffer(pH = 6.0) |
| CD-163 (bs-2527R, Bioss, MA, USA) | 1:1000 | EDTA buffer(pH = 9.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Maawi, S.; Wang, X.; Sader, R.; Götz, W.; Motta, A.; Migliaresi, C.; Kirkpatrick, C.J.; Ghanaati, S. Multinucleated Giant Cells Induced by a Silk Fibroin Construct Express Proinflammatory Agents: An Immunohistological Study. Materials 2021, 14, 4038. https://doi.org/10.3390/ma14144038
Al-Maawi S, Wang X, Sader R, Götz W, Motta A, Migliaresi C, Kirkpatrick CJ, Ghanaati S. Multinucleated Giant Cells Induced by a Silk Fibroin Construct Express Proinflammatory Agents: An Immunohistological Study. Materials. 2021; 14(14):4038. https://doi.org/10.3390/ma14144038
Chicago/Turabian StyleAl-Maawi, Sarah, Xuejiu Wang, Robert Sader, Werner Götz, Antonella Motta, Claudio Migliaresi, Charles James Kirkpatrick, and Shahram Ghanaati. 2021. "Multinucleated Giant Cells Induced by a Silk Fibroin Construct Express Proinflammatory Agents: An Immunohistological Study" Materials 14, no. 14: 4038. https://doi.org/10.3390/ma14144038