Thiol-Functionalized Ethylene Periodic Mesoporous Organosilica as an Efficient Scavenger for Palladium: Confirming the Homogeneous Character of the Suzuki Reaction
Abstract
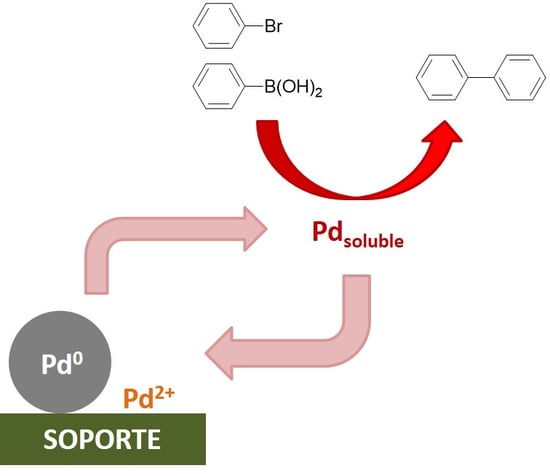
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Synthesis of the Catalysts
2.3. Characterization of the Catalysts
2.4. Catalytic Activity
2.4.1. General Procedure
2.4.2. Hot Filtration Tests
2.4.3. Catalyst Poisoning Tests
3. Results
3.1. Characterization of the Catalysts
3.2. Catalytic Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Inagaki, S.; Guan, S.; Fukushima, Y.; Ohsuna, T.; Terasaki, O. Novel Mesoporous Materials with a Uniform Distribution of Organic Groups and Inorganic Oxide in Their Frameworks. J. Am. Chem. Soc. 1999, 121, 9611–9614. [Google Scholar] [CrossRef]
- Asefa, T.; MacLachlan, M.J.; Coombs, N.; Ozin, G.A. Periodic mesoporous organosilicas with organic groups inside the channel walls. Nature 1999, 402, 867–871. [Google Scholar] [CrossRef]
- Melde, B.J.; Holland, B.T.; Blanford, C.F.; Stein, A. Mesoporous Sieves with Unified Hybrid Inorganic/Organic Frameworks. Chem. Mater. 1999, 11, 3302–3308. [Google Scholar] [CrossRef]
- Van Der Voort, P.; Esquivel, D.; De Canck, E.; Goethals, F.; Van Driessche, I.; Romero-Salguero, F.J. Periodic mesoporous organosilicas: From simple to complex bridges; A comprehensive overview of functions, morphologies and applications. Chem. Soc. Rev. 2013, 42, 3913–3955. [Google Scholar] [CrossRef]
- Hoffmann, F.; Cornelius, M.; Morell, J.; Fröba, M. Silica-based mesoporous organic-inorganic hybrid materials. Angew. Chem. Int. Ed. 2006, 45, 3216–3251. [Google Scholar] [CrossRef]
- Hao, N.; Han, L.; Yang, Y.; Wang, H.; Webley, P.A.; Zhao, D. A metal-ion-assisted assembly approach to synthesize disulfide-bridged periodical mesoporous organosilicas with high sulfide contents and efficient adsorption. Appl. Surf. Sci. 2010, 256, 5334–5342. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, W.; Shi, J.; Hua, Z.; Li, Y.; Yan, J. A new thioether functionalized organic-inorganic mesoporous composite as a highly selective and capacious Hg2+ adsorbent. Chem. Commun. 2003, 9, 210–211. [Google Scholar] [CrossRef]
- Liu, J.; Yang, J.; Yang, Q.; Wang, G.; Li, Y. Hydrothermally Stable Thioether-Bridged Mesoporous Materials with Void Defects in the Pore Walls. Adv. Funct. Mater. 2005, 15, 1297–1302. [Google Scholar] [CrossRef]
- Imamoğlu, M.; Pérez-Quintanilla, D.; Sierra, I. Bifunctional periodic mesoporous organosilicas with sulfide bridges as effective sorbents for Hg(II) extraction from environmental and drinking waters. Microporous Mesoporous Mater. 2016, 229, 90–97. [Google Scholar] [CrossRef]
- Olkhovyk, O.; Pikus, S.; Jaroniec, M.; Pikus, S. Bifunctional periodic mesoporous organosilica with large heterocyclic bridging groups and mercaptopropyl ligands. J. Mater. Chem. 2005, 15, 1517. [Google Scholar] [CrossRef]
- Wu, H.-Y.; Chen, C.-T.; Hung, I.-M.; Liao, C.-H.; Vetrivel, S.; Kao, H.-M. Direct Synthesis of Cubic Benzene-Bridged Mesoporous Organosilica Functionalized with Mercaptopropyl Groups as an Effective Adsorbent for Mercury and Silver Ions. J. Phys. Chem. C 2010, 114, 7021–7029. [Google Scholar] [CrossRef]
- Zhang, W.-H.; Zhang, X.; Zhang, L.; Schroeder, F.; Harish, P.; Hermes, S.; Shi, J.; Fischer, R.A. Synthesis of periodic mesoporous organosilicas with chemically active bridging groups and high loadings of thiol groups. J. Mater. Chem. 2007, 17, 4320. [Google Scholar] [CrossRef]
- Esquivel, D.; Berg, O.V.D.; Romero-Salguero, F.J.; Du Prez, F.; Van Der Voort, P. 100% thiol-functionalized ethylene PMOs prepared by “thiol acid–ene” chemistry. Chem. Commun. 2013, 49, 2344–2346. [Google Scholar] [CrossRef] [PubMed]
- Esquivel, D.; Ouwehand, J.; Meledina, M.; Turner, S.; Van Tendeloo, G.; Romero-Salguero, F.J.; De Clercq, J.; Van Der Voort, P. Thiol-ethylene bridged PMO: A high capacity regenerable mercury adsorbent via intrapore mercury thiolate crystal formation. J. Hazard. Mater. 2017, 339, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Rostamnia, S.; Rahmani, T. Ordered mesoporous SBA-15/PrSO3Pd and SBA-15/PrSO3PdNP as active, reusable and selective phosphine-free catalysts in C-X activation Heck coupling process. Appl. Organomet. Chem. 2015, 29, 471–474. [Google Scholar] [CrossRef]
- Piao, Y.; Jang, Y.; Shokouhimehr, M.; Lee, I.S.; Hyeon, T.; Lee, J. Facile Aqueous-Phase Synthesis of Uniform Palladium Nanoparticles of Various Shapes and Sizes. Small 2007, 3, 255–260. [Google Scholar] [CrossRef]
- Sydnes, M.O. The Use of Palladium on Magnetic Support as Catalyst for Suzuki–Miyaura Cross-Coupling Reactions. Catalysts 2017, 7, 35. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Kim, J.W.; Shokouhimehr, M.; Lee, Y.S. Polymer-supported N-heterocyclic carbene-palladium complex for heterogeneous suzuki cross-coupling reaction. J. Org. Chem. 2005, 70, 6714–6720. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Lee, J.E.; Han, S.I.; Hyeon, T. Magnetically recyclable hollow nanocomposite catalysts for heterogeneous reduction of nitroarenes and Suzuki reactions. Chem. Commun. 2013, 49, 4779–4781. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, A. Recent advances in the cross-coupling reactions of organoboron derivatives with organic electrophiles, 1995–1998. J. Organomet. Chem. 1999, 576, 147–168. [Google Scholar] [CrossRef]
- Grasa, G.A.; Viciu, M.S.; Huang, J.; Zhang, C.; Trudell, M.L.; Nolan, S.P. Suzuki−Miyaura Cross-Coupling Reactions Mediated by Palladium/Imidazolium Salt Systems. Organometallics 2002, 21, 2866–2873. [Google Scholar] [CrossRef]
- Littke, A.F.; Fu, G.C. Palladium-Catalyzed Coupling Reactions of Aryl Chlorides. Angew. Chem. Int. Ed. 2002, 41, 4176–4211. [Google Scholar] [CrossRef]
- Zapf, A.; Ehrentraut, A.; Beller, M. A New Highly Efficient Catalyst System for the Coupling of Nonactivated and Deactivated Aryl Chlorides with Arylboronic Acids. Angew. Chem. Int. Ed. 2000, 39, 4153–4155. [Google Scholar] [CrossRef]
- Korolev, D.N.; Bumagin, N.A. An improved protocol for ligandless Suzuki-Miyaura coupling in water. Tetrahedron Lett. 2006, 47, 4225–4229. [Google Scholar] [CrossRef]
- Phan, N.T.S.; Van Der Sluys, M.; Jones, C.W. On the Nature of the Active Species in Palladium Catalyzed Mizoroki–Heck and Suzuki–Miyaura Couplings—Homogeneous or Heterogeneous Catalysis, a Critical Review. Adv. Synth. Catal. 2006, 348, 609–679. [Google Scholar] [CrossRef]
- Garrett, C.E.; Prasad, K. The Art of Meeting Palladium Specifications in Active Pharmaceutical Ingredients Produced by Pd-Catalyzed Reactions. Adv. Synth. Catal. 2004, 346, 889–900. [Google Scholar] [CrossRef]
- Königsberger, K.; Chen, G.-P.; Wu, R.R.; Girgis, M.J.; Prasad, K.; Repič, O.; Blacklock, T.J. A Practical Synthesis of 6-[2-(2,5-Dimethoxyphenyl)ethyl]-4-ethylquinazoline and the Art of Removing Palladium from the Products of Pd-Catalyzed Reactions. Org. Process. Res. Dev. 2003, 7, 733–742. [Google Scholar] [CrossRef]
- Richardson, J.M.; Jones, C.W. Poly(4-vinylpyridine) and Quadrapure TU as Selective Poisons for Soluble Catalytic Species in Palladium-Catalyzed Coupling Reactions – Application to Leaching from Polymer-Entrapped Palladium. Adv. Synth. Catal. 2006, 348, 1207–1216. [Google Scholar] [CrossRef]
- Broadwater, S.J.; McQuade, D.T. Investigating PdEnCat Catalysis. J. Org. Chem. 2006, 71, 2131–2134. [Google Scholar] [CrossRef]
- Corma, A.; Das, D.; García, H.; Leyva, A. A periodic mesoporous organosilica containing a carbapalladacycle complex as heterogeneous catalyst for Suzuki cross-coupling. J. Catal. 2005, 229, 322–331. [Google Scholar] [CrossRef]
- Gruber-Woelfler, H.; Radaschitz, P.; Feenstra, P.; Haas, W.; Khinast, J. Synthesis, catalytic activity, and leaching studies of a heterogeneous Pd-catalyst including an immobilized bis(oxazoline) ligand. J. Catal. 2012, 286, 30–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, R.L.; He, W.; Gebbink, R.J.M.K.; De Jong, K.P. Palladium nanoparticles confined in thiol-functionalized ordered mesoporous silica for more stable Heck and Suzuki catalysts. Catal. Sci. Technol. 2015, 5, 1919–1928. [Google Scholar] [CrossRef]
- Biffis, A.; Zecca, M.; Basato, M. Palladium metal catalysts in Heck C-C coupling reactions. J. Mol. Catal. A Chem. 2001, 173, 249–274. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Cheprakov, A.V. The Heck Reaction as a Sharpening Stone of Palladium Catalysis. Chem. Rev. 2000, 100, 3009–3066. [Google Scholar] [CrossRef]
- Gruber, A.S.; Pozebon, D.; Monteiro, A.L.; Dupont, J. On the use of phosphine-free PdCl2(SEt2)2 complex as catalyst precursor for the Heck reaction. Tetrahedron Lett. 2001, 42, 7345–7348. [Google Scholar] [CrossRef]
- De Vries, A.H.M.; Mulders, J.M.C.A.; Mommers, J.H.M.; Henderickx, H.J.W.; de Vries, J.G. Homeopathic ligand-free palladium as a catalyst in the heck reaction. A comparison with a palladacycle. Org. Lett. 2003, 5, 3285–3288. [Google Scholar] [CrossRef]
- Arvela, R.K.; Leadbeater, N.E.; Sangi, M.S.; Williams, V.A.; Granados, P.; Singer, R.D. A Reassessment of the Transition-Metal Free Suzuki-Type Coupling Methodology. J. Org. Chem. 2005, 70, 161–168. [Google Scholar] [CrossRef]
- Zhao, F.; Shirai, M.; Ikushima, Y.; Arai, M. The leaching and re-deposition of metal species from and onto conventional supported palladium catalysts in the Heck reaction of iodobenzene and methyl acrylate in N-methylpyrrolidone. J. Mol. Catal. A Chem. 2002, 180, 211–219. [Google Scholar] [CrossRef]
- Zhao, F.; Murakami, K.; Shirai, M.; Arai, M. Recyclable Homogeneous/Heterogeneous Catalytic Systems for Heck Reaction through Reversible Transfer of Palladium Species between Solvent and Support. J. Catal. 2000, 194, 479–483. [Google Scholar] [CrossRef]
- Schmidt, A.F.; Smirnov, V.V. Simple method for enhancement of the ligand-free palladium catalyst activity in the Heck reaction with non-activated bromoarenes. J. Mol. Catal. A Chem. 2003, 203, 75–78. [Google Scholar] [CrossRef]
- Gruttadauria, M.; Giacalone, F.; Noto, R. “Release and catch” catalytic systems. Green Chem. 2013, 15, 2608–2618. [Google Scholar] [CrossRef]
- Vercaemst, C.; Ide, M.; Allaert, B.; Ledoux, N.; Verpoort, F.; Van Der Voort, P. Ultra-fast hydrothermal synthesis of diastereoselective pure ethenylene-bridged periodic mesoporous organosilicas. Chem. Commun. 2007, 22, 2261–2263. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.I.; Esquivel, D.; Jiménez-Sanchidrián, C.; Romero-Salguero, F.J.; Van Der Voort, P. A “one-step” sulfonic acid PMO as a recyclable acid catalyst. J. Catal. 2015, 326, 139–148. [Google Scholar] [CrossRef]
- Webb, J.; MacQuarrie, S.; McEleney, K.; Crudden, C. Mesoporous silica-supported Pd catalysts: An investigation into structure, activity, leaching and heterogeneity. J. Catal. 2007, 252, 97–109. [Google Scholar] [CrossRef]
- Burleigh, M.C.; Jayasundera, S.; Thomas, C.W.; Spector, M.S.; Markowitz, M.A.; Gaber, B.P. A versatile synthetic approach to periodic mesoporous organosilicas. Colloid Polym. Sci. 2004, 282, 728–733. [Google Scholar] [CrossRef]
- Corral, J.A.; López, M.I.; Esquivel, L.; Mora, M.; Jiménez-Sanchidrián, C.; Romero-Salguero, F.J. Preparation of Palladium-Supported Periodic Mesoporous Organosilicas and their Use as Catalysts in the Suzuki Cross-Coupling Reaction. Materials 2013, 6, 1554–1565. [Google Scholar] [CrossRef] [Green Version]
- Le, X.; Dong, Z.; Jin, Z.; Wang, Q.; Ma, J. Suzuki–Miyaura cross-coupling reactions catalyzed by efficient and recyclable Fe3O4@SiO2@mSiO2–Pd(II) catalyst. Catal. Commun. 2014, 53, 47–52. [Google Scholar] [CrossRef]
- Esquivel, D.; Jiménez-Sanchidrián, C.; Romero-Salguero, F.J. Comparison of the thermal and hydrothermal stabilities of ethylene, ethylidene, phenylene and biphenylene bridged periodic mesoporous organosilicas. Mater. Lett. 2011, 65, 1460–1462. [Google Scholar] [CrossRef]
- Khan, H.; Badshah, A.; Murtaz, G.; Said, M.; Rehman, Z.-U.-; Neuhausen, C.; Todorova, M.; Jean-Claude, B.J.; Butler, I.S. Synthesis, characterization and anticancer studies of mixed ligand dithiocarbamate palladium(II) complexes. Eur. J. Med. Chem. 2011, 46, 4071–4077. [Google Scholar] [CrossRef]
- Widegren, J.A.; Finke, R.G. A review of the problem of distinguishing true homogeneous catalysis from soluble or other metal-particle heterogeneous catalysis under reducing conditions. J. Mol. Catal. A Chem. 2003, 198, 317–341. [Google Scholar] [CrossRef]
- Richardson, J.M.; Jones, C.W. Strong evidence of solution-phase catalysis associated with palladium leaching from immobilized thiols during Heck and Suzuki coupling of aryl iodides, bromides, and chlorides. J. Catal. 2007, 251, 80–93. [Google Scholar] [CrossRef]
- El Kadib, A.; McEleney, K.; Seki, T.; Wood, T.K.; Crudden, C.M. Cross-Coupling in the Preparation of Pharmaceutically Relevant Substrates using Palladium Supported on Functionalized Mesoporous Silicas. Chem. Cat. Chem. 2011, 3, 1281–1285. [Google Scholar] [CrossRef]










| Material | a0 (Å) 1 | BET Surface Area (m2 g−1) 2 | Pore Volume (cm3 g−1) 3 | Pore Diameter (Å) 4 | Wall Thickness (Å) 5 |
|---|---|---|---|---|---|
| SH-PMO | 96 | 448 | 0.49 | 45 | 51 |
| Pd-SH-PMO | 87 | 202 | 0.22 | 36 | 51 |
| Pd-SH-PMO(H2) | 81 | 211 | 0.22 | 34 | 47 |
| SH-E-PMO | 102 | 637 | 0.61 | 66 | 36 |
| Pd-SH-E-PMO | 100 | 269 | 0.31 | 58 | 42 |
| Pd-SH-E-PMO(H2) | 92 | 291 | 0.32 | 54 | 38 |
| Material | Composition (wt%) 1 | C/S Atomic Ratio | Pd (wt%) 2 | Pd Incorporated (%) | S/Pd Atomic Ratio | S Atoms /nm2 | |
|---|---|---|---|---|---|---|---|
| C | S | ||||||
| SH-PMO | 16.90 | 13.89 | 3.25 | - | - | - | 5.8 |
| Pd-SH-PMO | 11.80 | 11.99 | 2.63 | 5.32 | 23.4 | 7.5 | 11.1 |
| Pd-SH-PMO(H2) | 11.02 | 12.05 | 2.44 | 4.47 | 19.6 | 9.0 | 10.7 |
| SH-E-PMO | 18.36 | 6.79 | 7.22 | - | - | - | 2.0 |
| Pd-SH-E-PMO | 15.77 | 5.70 | 7.39 | 2.34 | 22.84 | 8.1 | 4.0 |
| Pd-SH-E-PMO(H2) | 13.87 | 4.72 | 7.84 | 2.13 | 20.79 | 7.4 | 3.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, M.I.; Esquivel, D.; Jiménez-Sanchidrián, C.; Van Der Voort, P.; Romero-Salguero, F.J. Thiol-Functionalized Ethylene Periodic Mesoporous Organosilica as an Efficient Scavenger for Palladium: Confirming the Homogeneous Character of the Suzuki Reaction. Materials 2020, 13, 623. https://doi.org/10.3390/ma13030623
López MI, Esquivel D, Jiménez-Sanchidrián C, Van Der Voort P, Romero-Salguero FJ. Thiol-Functionalized Ethylene Periodic Mesoporous Organosilica as an Efficient Scavenger for Palladium: Confirming the Homogeneous Character of the Suzuki Reaction. Materials. 2020; 13(3):623. https://doi.org/10.3390/ma13030623
Chicago/Turabian StyleLópez, María I., Dolores Esquivel, César Jiménez-Sanchidrián, Pascal Van Der Voort, and Francisco J. Romero-Salguero. 2020. "Thiol-Functionalized Ethylene Periodic Mesoporous Organosilica as an Efficient Scavenger for Palladium: Confirming the Homogeneous Character of the Suzuki Reaction" Materials 13, no. 3: 623. https://doi.org/10.3390/ma13030623