Effect of Time-Dependent Characteristics of ZnO Nanoparticles Electron Transport Layer Improved by Intense-Pulsed Light Post-Treatment on Hole-Electron Injection Balance of Quantum-Dot Light-Emitting Diodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of HODs, EODs, and QLEDs
2.3. Characterization and Measurements
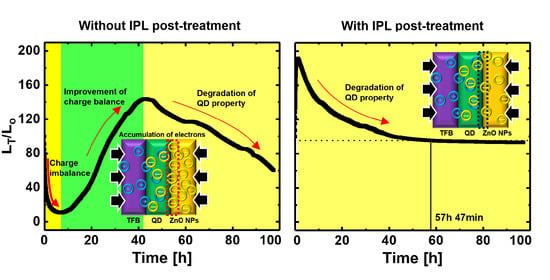
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Qian, L.; Zheng, Y.; Xue, J.; Holloway, P.H. Stable and efficient quantum-dot light-emitting diodes based on solution-processed multilayer structures. Nat. Photon. 2011, 5, 543–548. [Google Scholar] [CrossRef]
- Bae, W.K.; Park, Y.-S.; Lim, J.; Lee, D.; Padilha, L.A.; McDaniel, H.; Robel, I.; Lee, C.; Pietryga, J.M.; Klimov, V.I. Controlling the influence of Auger recombination on the performance of quantum-dot light-emitting diodes. Nat. Commun. 2013, 4, 2661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirasaki, Y.; Supran, G.J.; Bawendi, M.G.; Bulović, V. Emergence of colloidal quantum-dot light-emitting technologies. Nat. Photon. 2013, 7, 13–23. [Google Scholar] [CrossRef]
- Shen, H.; Gao, Q.; Zhang, Y.; Lin, Y.; Lin, Q.; Li, Z.; Chen, L.; Zeng, Z.; Li, X.; Jia, Y.; et al. Visible quantum dot light-emitting diodes with simultaneous high brightness and efficiency. Nat. Photon. 2019, 13, 192–197. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Wang, T.; Zhang, H.; Zhang, H.; Wang, R.; Ji, W. Efficient Structure for InP/ZnS-Based Electroluminescence Device by Embedding the Emitters in the Electron-Dominating Interface. J. Phys. Chem. Lett. 2020, 11, 1835–1839. [Google Scholar] [CrossRef] [PubMed]
- Hirose, T.; Tsunoi, T.; Aoyama, E.; Ishigaki, A.; Suzuki, T.; Inoue, H.; Nakashima, H.; Seo, S.; Tsutsui, T. 20-4: High-efficiency Perovskite QLED Achieving BT.2020 Green Chromaticity. SID Symp. Dig. Tech. Pap. 2017, 48, 284–287. [Google Scholar] [CrossRef]
- Pidluzhna, A.; Ivaniuk, K.; Stakhira, P.; Hotra, Z.; Chapran, M.; Ulanski, J.; Tynkevych, O.; Khalavka, Y.; Baryshnikov, G.; Minaev, B.; et al. Multi-channel electroluminescence of CdTe/CdS core-shell quantum dots implemented into a QLED device. Dye. Pigment. 2019, 162, 647–653. [Google Scholar] [CrossRef]
- Jiang, C.; Zhong, Z.; Liu, B.; He, Z.; Zou, J.; Wang, L.; Wang, J.; Peng, J.; Cao, Y. Coffee-Ring-Free Quantum Dot Thin Film Using Inkjet Printing from a Mixed-Solvent System on Modified ZnO Transport Layer for Light-Emitting Devices. ACS Appl. Mater. Interfaces 2016, 8, 26162–26168. [Google Scholar] [CrossRef]
- Kim, H.-M.; Youn, J.-H.; Seo, G.-J.; Jang, J. Inverted quantum-dot light-emitting diodes with solution-processed aluminium–zinc oxide as a cathode buffer. J. Mater. Chem. C 2013, 1, 1567–1573. [Google Scholar] [CrossRef]
- Pan, J.; Chen, J.; Huang, Q.; Khan, Q.; Liu, X.; Tao, Z.; Zhang, Z.; Lei, W.; Nathan, A. Size Tunable ZnO Nanoparticles To Enhance Electron Injection in Solution Processed QLEDs. ACS Photon. 2016, 3, 215–222. [Google Scholar] [CrossRef]
- Masuda, S.; Kitamura, K.; Okumura, Y.; Miyatake, S.; Tabata, H.; Kawai, T. Transparent thin film transistors using ZnO as an active channel layer and their electrical properties. J. Appl. Phys. 2003, 93, 1624–1630. [Google Scholar] [CrossRef]
- Fortunato, E.M.C.; Barquinha, P.M.C.; Pimentel, A.C.M.B.G.; Gonçalves, A.M.F.; Marques, A.J.S.; Pereira, L.M.N.; Martins, R.F.P. Fully Transparent ZnO Thin-Film Transistor Produced at Room Temperature. Adv. Mater. 2005, 17, 590–594. [Google Scholar] [CrossRef]
- Moghaddam, A.B.; Nazari, T.; Badraghi, J.; Kazemzad, M. Synthesis of ZnO Nanoparticles and Electrodeposition of Polypyrrole/ZnO Nanocomposite Film. Int. J. Electrochem. Sci. 2009, 4, 247–257. [Google Scholar]
- Wahab, R.; Mishra, A.; Yun, S.-I.; Kim, Y.-S.; Shin, H.-S. Antibacterial activity of ZnO nanoparticles prepared via non-hydrolytic solution route. Appl. Microbiol. Biotechnol. 2010, 87, 1917–1925. [Google Scholar] [CrossRef]
- Wu, X.L.; Siu, G.G.; Fu, C.L.; Ong, H.C. Photoluminescence and cathodoluminescence studies of stoichiometric and oxygen-deficient ZnO films. Appl. Phys. Lett. 2001, 78, 2285–2287. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Zhang, Z.; Jin, Y.; Niu, Y.; Cao, H.; Liang, X.; Chen, L.; Wang, J.; Peng, X. Solution-processed, high-performance light-emitting diodes based on quantum dots. Nat. Cell Biol. 2014, 515, 96–99. [Google Scholar] [CrossRef]
- Jin, X.; Chang, C.; Zhao, W.; Huang, S.; Gu, X.; Zhang, Q.; Li, F.; Zhang, Y.; Li, Q. Balancing the Electron and Hole Transfer for Efficient Quantum Dot Light-Emitting Diodes by Employing a Versatile Organic Electron-Blocking Layer. ACS Appl. Mater. Interfaces 2018, 10, 15803–15811. [Google Scholar] [CrossRef]
- Shan, F.K.; Kim, B.I.; Liu, G.X.; Liu, Z.F.; Sohn, J.Y.; Lee, W.J.; Shin, B.C.; Yu, Y.S. Blueshift of near band edge emission in Mg doped ZnO thin films and aging. J. Appl. Phys. 2004, 95, 4772–4776. [Google Scholar] [CrossRef]
- Shan, F.K.; Liu, G.X.; Lee, W.J.; Lee, G.H.; Kim, I.S.; Shin, B.C. Aging effect and origin of deep-level emission in ZnO thin film deposited by pulsed laser deposition. Appl. Phys. Lett. 2005, 86, 221910. [Google Scholar] [CrossRef]
- Hak-SungKim, H.-S.; Dhage, S.R.; Shim, D.-E.; Hahn, H.T. Intense pulsed light sintering of copper nanoink for printed electronics. Appl. Phys. A 2009, 97, 791–798. [Google Scholar] [CrossRef] [Green Version]
- Perelaer, J.; Abbel, R.; Wünscher, S.; Jani, R.; Van Lammeren, T.; Schubert, U.S. Roll-to-Roll Compatible Sintering of Inkjet Printed Features by Photonic and Microwave Exposure: From Non-Conductive Ink to 40% Bulk Silver Conductivity in Less Than 15 Seconds. Adv. Mater. 2012, 24, 2620–2625. [Google Scholar] [CrossRef]
- Niittynen, J.; Sowade, E.; Kang, H.; Baumann, R.R.; Mäntysalo, M. Comparison of laser and intense pulsed light sintering (IPL) for inkjet-printed copper nanoparticle layers. Sci. Rep. 2015, 5, 8832. [Google Scholar] [CrossRef]
- Greenberg, B.L.; Robinson, Z.L.; Reich, K.V.; Gorynski, C.; Voigt, B.N.; Francis, L.F.; Shklovskii, B.I.; Aydil, E.S.; Kortshagen, U.R. ZnO Nanocrystal Networks Near the Insulator–Metal Transition: Tuning Contact Radius and Electron Density with Intense Pulsed Light. Nano Lett. 2017, 17, 4634–4642. [Google Scholar] [CrossRef] [Green Version]
- Kathirgamanathan, P.; Kumaraverl, M.; Vanga, R.R.; Ravichandran, S. Intense pulsed light (IPL) annealed sol–gel derived ZnO electron injector for the production of high efficiency inverted quantum dot light emitting devices (QLEDs). RSC Adv. 2018, 8, 36632–36646. [Google Scholar] [CrossRef] [Green Version]
- Moon, C.-J.; Jeong, S.H. Intense Pulsed Light Annealing Process of Indium–Gallium–Zinc–Oxide Semiconductors via Flash White Light Combined with Deep-UV and Near-Infrared Drying for High-Performance Thin-Film Transistors. ACS Appl. Mater. Interfaces 2019, 11, 13380–13388. [Google Scholar] [CrossRef]
- Han, Y.J.; An, K.; Kang, K.T.; Ju, B.-K.; Cho, K.H. Optical and Electrical Analysis of Annealing Temperature of High-Molecular Weight Hole Transport Layer for Quantum-dot Light-emitting Diodes. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-Ray Photoelectron Spectroscopy; Physical Electronics: Eden Prairie, MN, USA, 1995. [Google Scholar]
- Zhang, Q.; Xu, M.; You, B.; Zhang, Q.; Yuan, H.; Ostrikov, K. (Ken) Oxygen Vacancy-Mediated ZnO Nanoparticle Photocatalyst for Degradation of Methylene Blue. Appl. Sci. 2018, 8, 353. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, P.-T.; Chen, Y.-C.; Kao, K.-S.; Wang, C.-M. Luminescence mechanism of ZnO thin film investigated by XPS measurement. Appl. Phys. A 2007, 90, 317–321. [Google Scholar] [CrossRef]
- Pei, Z.; Ding, L.; Hu, J.; Weng, S.; Zheng, Z.; Huang, M.; Liu, P. Defect and its dominance in ZnO films: A new insight into the role of defect over photocatalytic activity. Appl. Catal. B Environ. 2013, 736–743. [Google Scholar] [CrossRef]
- Hikmet, R.R.M.; Talapin, D.; Weller, H. Study of conduction mechanism and electroluminescence in CdSe/ZnS quantum dot composites. J. Appl. Phys. 2003, 93, 3509. [Google Scholar] [CrossRef]
- Kim, S.-K.; Yang, H.; Kim, Y.-S. Control of carrier injection and transport in quantum dot light emitting diodes (QLEDs) via modulating Schottky injection barrier and carrier mobility. J. Appl. Phys. 2019, 126, 185702. [Google Scholar] [CrossRef]
- Hammad, T.M.; Salem, J.K.; Harrison, R.G. The influence of annealing temperature on the structure, morphologies and optical properties of ZnO nanoparticles. Superlattices Microstruct. 2010, 47, 335–340. [Google Scholar] [CrossRef]
- Swarnkar, A.; Chulliyil, R.; Ravi, V.K.; Irfanullah, M.; Chowdhury, A.; Nag, A. Colloidal CsPbBr3Perovskite Nanocrystals: Luminescence beyond Traditional Quantum Dots. Angew. Chem. 2015, 127, 15644–15648. [Google Scholar] [CrossRef]
- Yuan, X.; Hou, X.; Li, J.; Qu, C.; Zhang, W.; Zhao, J.; Li, H. Thermal degradation of luminescence in inorganic perovskite CsPbBr3 nanocrystals. Phys. Chem. Chem. Phys. 2017, 19, 8934–8940. [Google Scholar] [CrossRef]





| QLEDs without IPL | QLEDs with IPL | ||
|---|---|---|---|
| Luminance (cd/m2) | Initial: | 103,746 | 110,794 |
| After 8 Day: | 97,554 | 92,963 | |
| CE (cd/A) | Initial: | 21.565 | 24.3235 |
| After 8 Day: | 26.998 | 25.8653 | |
| EQE (%) | Initial: | 5.657 | 6.159 |
| After 8 Day: | 7.220 | 6.801 | |
| Stabilization Time (Day) | 1-day | Almost Initial | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.J.; Kang, K.-T.; Ju, B.-K.; Cho, K.H. Effect of Time-Dependent Characteristics of ZnO Nanoparticles Electron Transport Layer Improved by Intense-Pulsed Light Post-Treatment on Hole-Electron Injection Balance of Quantum-Dot Light-Emitting Diodes. Materials 2020, 13, 5041. https://doi.org/10.3390/ma13215041
Han YJ, Kang K-T, Ju B-K, Cho KH. Effect of Time-Dependent Characteristics of ZnO Nanoparticles Electron Transport Layer Improved by Intense-Pulsed Light Post-Treatment on Hole-Electron Injection Balance of Quantum-Dot Light-Emitting Diodes. Materials. 2020; 13(21):5041. https://doi.org/10.3390/ma13215041
Chicago/Turabian StyleHan, Young Joon, Kyung-Tae Kang, Byeong-Kwon Ju, and Kwan Hyun Cho. 2020. "Effect of Time-Dependent Characteristics of ZnO Nanoparticles Electron Transport Layer Improved by Intense-Pulsed Light Post-Treatment on Hole-Electron Injection Balance of Quantum-Dot Light-Emitting Diodes" Materials 13, no. 21: 5041. https://doi.org/10.3390/ma13215041