A Novel Green Extraction Technique for Extracting Flavonoids from Folium nelumbinis by Changing Osmosis Pressure
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. Apparatus
2.3. Chromatographic Conditions
2.4. Standard Solution Preparation
2.5. Flavonoid Extraction Method
2.5.1. Osmosis Extraction
2.5.2. Ultrasonication-Assisted Osmosis Extraction (OE)
2.5.3. Single-Phase Extraction
2.5.4. Ultrasonication-Assisted Single-Phase Extraction
2.6. Total Flavonoid Content Determination
2.7. Antioxidant Activity Against 2,2-Diphenyl-1-Picrylhydrazyl Radicals
2.8. Statistical Analysis
3. Results and Discussion
3.1. Validation of the Analytical Methods
3.2. Optimization of the OE Conditions
3.2.1. Effect of Ethanol Concentration
3.2.2. Effect of the Mass of Salt
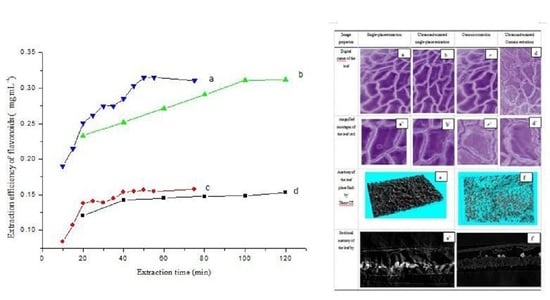
3.2.3. Kinetics of the Extraction Process
3.2.4. Mechanism of the Promotion of Dissolution by Inorganic Salts
3.3. Evaluation of the Proposed Separation Process
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wei, Y.Q.; Sun, M.M.; Fang, H.Y. Dienzyme-assisted salting-out extraction of flavonoids from the seeds of Cuscuta chinensis Lam. Ind. Crop. Prod. 2019, 127, 232–236. [Google Scholar] [CrossRef]
- Fu, H.; Yang, S.T.; Xiu, Z. Phase separation in a salting-out extraction system of ethanol-ammonium sulfate. Sep. Purif. Technol. 2015, 148, 32–37. [Google Scholar] [CrossRef]
- Hou, B.J.; Wei, Y.Q.; Ma, F.; Wang, X.N.; Yang, S.Z. Chelatometric salting-out extraction and characteristics of flavonoids from Folium nelumbinis based on an ethanol/K2HPO4 system. Sep. Sci. Technol. 2017, 12, 717–724. [Google Scholar] [CrossRef]
- Reis, I.A.; Santos, S.B.; Pereira, F.D.; Sobral, C.R.; Freire, M.G.; Freitas, L.S.; Soares, C.M.; Lima, A.S. Extraction and recovery of rutin from acerola waste using alcohol-salt-based aqueous two-phase systems. Sep. Purif. Technol. 2014, 49, 656–663. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, D.; Fan, H.; Liu, X.; Wan, Q.; Wu, X.; Liu, P.; Tang, J.Z. Simultaneous extraction and purification of alkaloids from Sophora flavescens Ait. by microwave-assisted aqueous two-phase extraction with ethanol/ammonia sulfate system. Sep. Purif. Technol. 2015, 141, 113–123. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, W.; Tang, X.; Fan, H.; Xie, X.; Wan, Q.; Wu, X.; Tang, J.Z. Extraction and characterization of polysaccharides from Semen Cassiae by microwave-assisted aqueous two-phase extraction coupled with spectroscopy and HPLC. Carbohyd. Polym. 2016, 144, 263–270. [Google Scholar] [CrossRef]
- Guo, T.; Su, D.; Huang, Y.; Wang, Y.; Li, Y.H. Ultrasound-assisted aqueous two-phase system for extraction and enrichment of Zanthoxylum armatum lignans. Molecules 2015, 20, 15273–15286. [Google Scholar] [CrossRef]
- Dong, B.; Yuan, X.; Zhao, Q.; Feng, Q.; Zhao, B. Ultrasound-assisted aqueous two-phase extraction of phenylethanoid glycosides from Cistanche deserticola Y. C. Ma stems. J. Sep. Sci. 2015, 38, 1194–1203. [Google Scholar] [CrossRef]
- Li, L.J.; Jin, Y.R.; Wang, X.Z.; Liu, Y.; Wu, Q.; Shi, X.L. Ionic liquid and aqueous two-phase extraction based on salting-out coupled with high-performance liquid chromatography for the determination of seven rare ginsenosides in Xue-Sai-Tong injection. J. Sep. Sci. 2015, 38, 3055–3062. [Google Scholar] [CrossRef]
- Feng, Y.C.; Li, W.L.; He, F.M.; Kong, T.T.; Huang, X.W.; Gao, Z.H.; Lu, N.H.; Li, H.L. Aqueous two-phase system as an effective tool for purification of phenolic compounds from fig fruits (Ficus carica L.). Sep. Purif. Technol. 2015, 50, 1785–1793. [Google Scholar]
- Wei, Y.Q.; Hou, B.J.; Fang, H.Y.; Sun, X.J.; Ma, F. Salting-out extraction of ginsenosides from the enzymatic hydrolysates of Panax quinquefolium based on ethanol/sodium carbonate system. J. Ginseng Res. 2020, 1, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yan, L.; Fu, H.; Xiu, Z. Salting-out extraction and crystallization of succinic acid from fermentation broths. Process. Biochem. 2014, 49, 506–511. [Google Scholar] [CrossRef]
- Ooi, C.W.; Tey, B.T.; Hii, S.L.; Kamal, S.M.M.; Lan, J.C.W.; Ariff, A.; Ling, T.C. Purification of lipase derived from Burkholderia pseudomallei with alcohol/saltbased aqueous two-phase systems. Process. Biochem. 2009, 44, 1083–1087. [Google Scholar] [CrossRef]
- Zhang, Z.R.; Shen, J.T.; Dai, J.Y.; Sun, Y.Q.; Xiu, Z.L. Separation and purification of klebsiella phage by two-step salting-out extraction. Sep. Purif. Technol. 2020. [Google Scholar] [CrossRef]
- Amid, M.; Shuhaimi, M.; Sarker, M.Z.I.; Manap, M.Y.A. Purification of serine protease from mango (Mangifera Indica Cv. Chokanan) peel using an alcohol/salt aqueous two phase system. Food Chem. 2012, 132, 1382–1386. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Wahile, A.; Mukherjee, K.; Saha, B.P.; Mukherjee, P.K. Antioxidant activity of Nelumbo nucifera (sacred lotus) seeds. J. Ethnopharmacol. 2006, 104, 322–327. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Jang, S.A.; Lee, A.; Cho, C.W.; Kim, T. Polysaccharidesisolated from lotus leaves (LLEP) exert anti-osteoporotic effects by inhibiting osteoclastogenesis. Int. J. Biol. Macromol. 2020. [Google Scholar] [CrossRef]
- Kashiwada, Y.; Aoshima, A.; Ikeshiro, Y.; Chen, Y.P.; Furukawa, H.; Itoigawa, M.; Fujioka, T.; Mihashi, K.; Cosentino, L.M.; Morris-Natschke, S.L.; et al. Anti-HIV benzylisoquinoline alkaloids and flavonoids from the leaves of Nelumbo nucifera, and structure-activity correlations with related alkaloids. Bioorg. Med. Chem. 2005, 13, 443–448. [Google Scholar] [CrossRef]
- Ohkoshi, E.; Miyazaki, H.; Shindo, K.; Watanabe, H.; Yoshida, A.; Yajima, H. Constituents from the leaves of Nelumbo nucifera stimulate lipolysis in the white adipose tissue of mice. Planta Med. 2007, 73, 1255–1259. [Google Scholar] [CrossRef] [Green Version]
- Je, J.Y.; Lee, D.B. Nelumbo nucifera leaves protect hydrogen peroxide-induced hepatic damage via antioxidant enzymes and HO-1/Nrf2 activation. Food Funct. 2015, 6, 1911–1918. [Google Scholar] [CrossRef]
- Song, Y.R.; Han, A.R.; Lim, T.G.; Lee, E.J.; Hong, H.D. Isolation, purification, and characterization of novel polysaccharides from lotus (Nelumbo nucifera) leaves and their immunostimulatory effects. Int. J. Biol. Macromol. 2019, 128, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.R.; Han, A.R.; Park, S.G.; Cho, C.W.; Rhee, Y.K.; Hong, H.D. Effect of enzyme-assisted extraction on the physicochemical properties and bioactive potential of lotus leaf polysaccharides. Int. J. Biol. Macromol. 2020, 153, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Chong, K.Y.; Stefanova, R.; Zhang, J.; Brooks, M.S.L. Aqueous two-phase extraction of bioactive compounds from haskap leaves (Lonicera caerulea): Comparison of salt/ethanol and sugar/propanol systems. Sep. Purif. Technol. 2020. [Google Scholar] [CrossRef]
- Yau, Y.K.; Ooi, C.W.; Ng, E.P.; Lan, J.C.W.; Ling, T.C.; Show, P.L. Current applications of 29 different type of aqueous two-phase systems. Bioresour. Bioprocess. 2015, 2, 1–13. [Google Scholar] [CrossRef] [Green Version]





| Sample No. | Ethanol (mL) | Volume Ratio | Absorption | Flavonoids (mg/L) |
|---|---|---|---|---|
| 1 | 4.0 | 1.714 | 0.492 | 0.043 |
| 2 | 3.8 | 2.000 | 0.577 | 0.050 |
| 3 | 3.5 | 1.375 | 0.375 | 0.0325 |
| 4 | 3.3 | 1.594 | 0.345 | 0.030 |
| 5 | 3.0 | 0.750 | 0.469 | 0.041 |
| Sample No. | Mass of Salt (g) | Volume Ratio | Absorption | Flavonoids (mg/L) |
|---|---|---|---|---|
| 1 | 1.6 | 1.771 | 0.253 | 0.044 |
| 2 | 1.7 | 2.214 | 0.276 | 0.048 |
| 3 | 1.8 | 2.000 | 0.307 | 0.054 |
| 4 | 1.9 | 1.400 | 0.321 | 0.056 |
| 5 | 2.0 | _ |
| Method | Yield (%) | PF of Flavonoids (%) | PF of Hyperoside (%) | IC50 (μg/mL) |
|---|---|---|---|---|
| This method | 4.20 | 85.71 | 61.11 | 6.4 |
| SOE method | 3.75 | 86.13 | 46.44 | 6.2 |
| Resin method | 3.49 | 86.53 | 47.35 | 6.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, H.-Y.; Wei, Y.-Q.; Zhang, M.-L.; Liu, W. A Novel Green Extraction Technique for Extracting Flavonoids from Folium nelumbinis by Changing Osmosis Pressure. Materials 2020, 13, 4192. https://doi.org/10.3390/ma13184192
Fang H-Y, Wei Y-Q, Zhang M-L, Liu W. A Novel Green Extraction Technique for Extracting Flavonoids from Folium nelumbinis by Changing Osmosis Pressure. Materials. 2020; 13(18):4192. https://doi.org/10.3390/ma13184192
Chicago/Turabian StyleFang, Hai-Yan, Ying-Qin Wei, Meng-Li Zhang, and Wei Liu. 2020. "A Novel Green Extraction Technique for Extracting Flavonoids from Folium nelumbinis by Changing Osmosis Pressure" Materials 13, no. 18: 4192. https://doi.org/10.3390/ma13184192