New Nanofibers Based on Protein By-Products with Bioactive Potential for Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
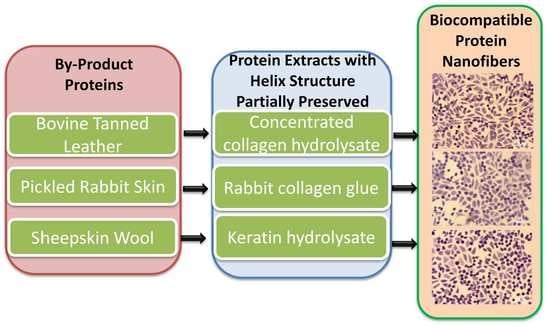
2.2. Preparation of HC10CC, RCG and KH Extracts
2.3. Electrospinning of HC10CC, RCG and KH Extracts
2.4. Characterization of HC10, HC10CC, RCG and KH Extracts
2.5. Characterization of Electrospun Protein-Based Nanofibers
2.5.1. Morphology and Structure
2.5.2. Differential Scanning Calorimetry (DSC)
2.5.3. Viscoelastic Properties
2.5.4. Biocompatibility Tests
2.6. Statistical Analysis
3. Results
3.1. Characterization of HC10, HC10CC, RCG and KH Extracts
3.2. Scanning Electron Microscopy with Energy Dispersive X-ray Spectroscopy (SEM-EDS)
3.3. Attenuated Total Reflection Fourier Transform Infrared (ATR-FTIR) Analysis
3.4. Differential Scanning Calorimetry (DSC)
3.5. Dynamic Nanoindentation Analysis
3.6. In Vitro Evaluation of Nanofibers’ Biocompatibility
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ibrahim, H.M.; Klingner, A. A review on electrospun polymeric nanofibers: Production parameters and potential applications. Polym. Test. 2020, 20, 106647. [Google Scholar] [CrossRef]
- Bombin, A.D.J.; Dunne, N.; McCarthy, H.O. Electrospinning of natural polymers for the production of nanofibres for wound healing applications. Mater. Sci. Eng. C 2020, 114, 110994. [Google Scholar] [CrossRef]
- Persano, L.; Camposeo, A.; Pisignano, D. Advancing the science and technology of electrospinning and functional nanofibers. Macromol. Mater. Eng. 2017, 302, 1700237. [Google Scholar] [CrossRef] [Green Version]
- Petropoulou, A.; Christodoulou, K.; Polydorou, C. Krasia-Christoforou, T.; Riziotis, C. Cost-Effective polymethacrylate-based electrospun fluorescent fibers toward ammonia sensing. Macromol. Mater. Eng. 2017, 302. [Google Scholar] [CrossRef]
- Wang, A.; Xu, C.; Zhang, C.; Wang, B. Experimental investigation of the properties of electrospun nanofibers for potential medical application. J. Nanomater. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Shekarforoush, E.; Faralli, A.; Ndoni, S.; Mendes, A.C.; Chronakis, I.S. Electrospinning of xanthan polysaccharide. Macromol. Mater. Eng. 2017, 302, 1700067. [Google Scholar] [CrossRef]
- Bayraktar, O. Silk fibroin nanofibers loaded with hydroxytyrosol from hydrolysis ofoleuropein in olive leaf extract. Text. Leather Rev. 2018, 1, 90–98. [Google Scholar] [CrossRef]
- Qi, P.; Zhou, Y.; Wang, D.; He, Z.; Li, Z. A new collagen solution with high concentration and collagennative structure perfectly preserved. RSC Adv. 2015, 5, 87180–87186. [Google Scholar] [CrossRef]
- Subtirica, A.I.; Chivu, A.A.M.; Banciu, C.A.; Dinca, L.C. Nanofibres made from biocompatible and biodegradable polymers, with potential application as medical textiles. Ind. Text. 2018, 69, 55–58. [Google Scholar]
- Harada, O. Preparation and characterization of collagen spun from liquid-crystallin collagen. In Proceedings of the 11th Asian International Conference of Leather Science and Technology, Xi’an, China, 16–19 October 2018; pp. 347–351. [Google Scholar]
- Bellini, D.; Cencetti, C.; Sacchetta, A.C.; Battista, A.M.; Martinelli, A.; Mazzucco, L.; Scotto D’Abusco, A.; Matricardi, P. PLA-Grafting of collagen chains leading to abiomaterial with mechanical performances useful in tendon regeneration. J. Mech. Behav. Biomed. Mater. 2016, 64, 151–160. [Google Scholar] [CrossRef]
- Ruszczaka, Z.; Friess, W. Collagen as a carrier for on-site delivery of antibacterialdrugs. Adv. Drug Deliv. Rev. 2003, 55, 1679–1698. [Google Scholar] [CrossRef] [PubMed]
- Bhuimbar, M.V.; Bhagwat, P.K.; Dandge, P.B. Extraction and characterization of acid soluble collagen from fish waste: Development of collagen-chitosan blend as food packaging film. J. Environ. Chem. Eng. 2019, 7, 102983. [Google Scholar] [CrossRef]
- Yorgancioglu, A.; Bayramoglu, E.E. Production of cosmetic purpose collagen containing antimicrobial emulsion with certain essential oils. Ind. Crops Prod. 2013, 44, 378–382. [Google Scholar] [CrossRef]
- Chai, H.J.; Li, J.H.; Huang, H.N.; Li, T.L.; Chan, Y.L.; Shiau, C.Y.; Wu, C.J. Effects of sizes and conformations of fish-scale collagen peptides on facial skin qualities and transdermal penetration efficiency. J. Biomed. Biotechnol. 2010, 2010, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arumugam, G.K.S.; Sharma, D.; Balakrishnan, R.M.; Ettiyappan, J.B.P. Extraction, optimization and characterization of collagen from sole fish skin. Sustain. Chem. Pharm. 2018, 9, 19–26. [Google Scholar] [CrossRef]
- Rapa, M.; Darie-Nita, R.N.; Preda, P.; Coroiu, V.; Tatia, R.; Vasile, C.; Matei, E.; Predescu, A.M.; Maxim, M.E. PLA/collagen hydrolysate/silver nanoparticles bionanocomposites for potential antimicrobial urinary drains. Polym. Plast. Technol. Mat. 2019, 58, 2041–2055. [Google Scholar] [CrossRef]
- Rapa, M.; Stefan, L.M.; Preda, P.; Darie-Nita, R.N.; Gaspar-Pintiliescu, A.; Seciu, A.M.; Vasile, C.; Matei, E.; Predescu, A.M. Effect of hydrolyzed collagen on thermal, mechanical and biological properties of poly(lactic acid) bionanocomposites. Iran. Polym. J. 2019, 28, 271–282. [Google Scholar] [CrossRef]
- Râpă, M.; Stefan, L.M.; Zaharescu, T.; Seciu, A.M.; Țurcanu, A.A.; Matei, E.; Predescu, A.M.; Antoniac, I.; Predescu, C. Development of bionanocomposites based on PLA, collagen and AgNPs and characterization of their stability and in vitro biocompatibility. Appl. Sci. 2020, 10, 2265. [Google Scholar] [CrossRef] [Green Version]
- Ghezzi, L.; Duce, C.; Bernazzani, L.; Bramanti, E.; Colombini, M.P.; Tiné, M.R.; Bonaduce, I. Interactions between inorganic pigments and rabbit skin glue in reference paint reconstructions. J. Therm. Anal. Calorim. 2015, 122, 315–322. [Google Scholar] [CrossRef]
- Becker, U.; Fietzek, P.P.; Furthmays, H.; Timpl, R. Non-Helical sequences of rabbit collagen—Correlation with antigenic determinants detected by rabbit antibodies in homologous regions of rat and calf collagen. Eur. J. Biochem. 1975, 54, 359–366. [Google Scholar] [CrossRef]
- Yu, W.; Wang, X.M.; Ma, L.; Li, H.J.; He, Z.F.; Zhang, Y.H. Preparation, characterisation and structure of rabbit (Hyla rabbit) skin gelatin. Int. J. Food Sci. Technol. 2016, 51, 574–580. [Google Scholar] [CrossRef]
- Tonin, C.; Aluigi, A.; Varesano, A.; Vineis, C. Nanofibers; Kumar, A., Ed.; InTech: Rijeka, Croatia, 2010; pp. 139–158. Available online: http://www.intechopen.com/books/nanofibers/keratin-based-nanofibres (accessed on 20 June 2020).
- Rouse, J.G.; Van Dyke, M.E. A review of keratin-based biomaterials for biomedical applications. Materials 2010, 3, 999–1014. [Google Scholar] [CrossRef] [Green Version]
- Spasova, M.; Manolova, N.; Paneva, D.; Rashkov, I. Preparation of chitosan-containing nanofibres by electrospinning of chitosan/poly(ethylene oxide) blend solutions. e-Polymers 2004, 4, 056. [Google Scholar] [CrossRef]
- Bahria, H. Electrospinning of collagen: Formation of biomedical scaffold. Adv. Res. Text. Eng. 2017, 2, 1017. [Google Scholar] [CrossRef]
- Chen, Z.; Mo, X.; Qing, F. Electrospinning of collagen–chitosan complex. Mat. Lett. 2007, 61, 3490–3494. [Google Scholar] [CrossRef]
- Jha, B.S.; Ayres, C.E.; Bowman, J.R.; Telemeco, T.A.; Sell, S.A.; Bowlin, G.L.; Simpson, D.G. Electrospun collagen: A tissue engineering scaffold with unique functional properties in a wide variety of applications. J. Nanomater. 2011, 2011, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Barrientos, I.J.H.; Paladino, E.; Szabó, P.; Brozio, S.; Hall, P.J.; Oseghale, C.I.; Passarelli, M.K.; Moug, S.J.; Black, R.A.; Wilson, C.G.; et al. Electrospun collagen-based nanofibres: A sustainable material for improved antibiotic utilisation in tissue engineering applications. Int. J. Pharm. 2017, 531, 67–79. [Google Scholar] [CrossRef] [Green Version]
- Zeugolis, D.I.; Khew, S.T.; Yew, E.S.Y.; Raghunath, M. Electro-Spinning of pure collagen nano-fibres—Just an expensive way to make gelatin? Biomaterials 2008, 29, 2293–2305. [Google Scholar] [CrossRef]
- Bürck, J.; Aras, O.; Bertinetti, L.; Ilhan, A.I.; Ermeydan, M.A.; Schneider, R.; Ulrich, S.; Kazanci, M. Observation of triple helix motif on electrospun collagen nanofibers and its effect on the physical and structural properties. J. Mol. Struct. 2018, 1151, 73–80. [Google Scholar] [CrossRef]
- Aluigi, A.; Veresano, A.; Montarsolo, A.; Vineis, C.; Ferrero, F.; Mazzuchetti, G.; Tonin, C. Electrospinning of keratin/poly(ethylene) oxide blend nanofibers. J. Appl. Polym. Sci. 2007, 104, 863–870. [Google Scholar] [CrossRef]
- Gaidau, C.; Ghiga, M.; Stepan, E.; Taloi, D.; Filipescu, L. Additives and advanced biomaterials obtained from leather industry by-products. Rev. Chim. Buchar. 2009, 60, 501–507. [Google Scholar]
- Gaidau, C.; Niculescu, M.D.; Epure, D.G.; Grzesiak, E.; Gendaszewska, D.; Lawinska, K.; Lason-Rydel, M.; Shalbuev, D. Composition Based on Collagen Hydrolysate and Method of Obtaining Thereof for Coating Leguminous Plant Seeds. EP 3488694A1, 20 May 2019. [Google Scholar]
- Berechet, M.D.; Niculescu, M.; Gaidau, C.; Ignat, M.; Epure, D.G. Alkaline-Enzymatic hydrolyses of wool waste for different applications. Rev. Chim. Buchar. 2018, 69, 1649–1654. [Google Scholar] [CrossRef]
- Gaidau, C.; Niculescu, M.; Epure, D.G.; Tudorache, M.; Radu, E.; Stoica, R.; Ignat, M.; Gidea, M. Collagen hydrolysate as sustainable additive for cereal seed treatment. Rev. Chim. Buchar. 2017, 68, 2783–2786. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. Measurement of hardness and elastic modulus by instrumented indentation: Advances in understanding and refinements to methodology. J. Mater. Res. 2004, 19, 3–20. [Google Scholar] [CrossRef]
- Herbert, E.G.; Oliver, W.C.; Pharr, G.M. Nanoindentation and the dynamic characterization of viscoelastic solids. J. Phys. D Appl. Phys. 2008, 41, 1–9. [Google Scholar] [CrossRef]
- Conte, N.; Jardret, V. Frequency specific characterization of very soft polymeric materials using nanoindentation testing. Mat. Res. Soc. Symp. Proc. 2002, 710, 1–6. [Google Scholar] [CrossRef]
- Hay, J.; Herbert, E. Measuring the complex modulus of polymers by instrumented indentation testing. Exp. Tech. 2011, 37, 55–61. [Google Scholar] [CrossRef]
- Craciunescu, O.; Gaspar, A.; Trif, M.; Moisei, M.; Oancea, A.; Moldovan, L.; Zarnescu, O. Preparation and characterization of a collagen-liposome-chondroitin sulfate matrix with potential application for inflammatory disorders treatment. J. Nanomater. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Allen, M.; Millett, P.; Dawes, E.; Rushton, N. Lactate dehydrogenase activity as a rapid and sensitive test for the quantification of cell numbers in vitro. Clin. Mater. 1994, 16, 189–194. [Google Scholar] [CrossRef]
- Brevern, A.G. Extension of the classical classification of β-turns. Sci. Rep. 2016, 6, 33191. [Google Scholar] [CrossRef]
- Krishnamoorthi, J.; Ramasamy, P.; Shanmugam, V.; Shanmugam, A. Isolation and partial characterization of collagen from outer skin of Sepia pharaonis (Ehrenberg, 1831) from Puducherry coast. Biochem. Biophys. Rep. 2017, 10, 39–45. [Google Scholar] [CrossRef]
- Wang, J.; Pei, X.; Liu, H.; Zhou, D. Extraction and characterization of acid-soluble and pepsin soluble collagen from skin of loach (Misgurnus anguillicaudatus). Int. J. Biol. Macromol. 2018, 106, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Jiang, W. Preparation of chitosan-based nanoparticles for enzyme immobilization. Int. J. Biol. Macromol. 2019, 126, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xie, G.; Su, Y.; Su, L.; Zhang, Q.; Du, H.; Tai, H.; Jiang, Y. Reduced graphene oxide-polyethylene oxide composite films for humidity sensing via quartz crystal microbalance. Sens. Actuators B Chem. 2018, 255, 2203–2210. [Google Scholar] [CrossRef]
- Fan, J.; Lei, T.D.; Li, J.; Zhai, P.Y.; Wang, Y.H.; Cao, F.Y.; Liu, Y. High protein content keratin/poly(ethylene) oxide nanofibers crosslinked in oxygen atmosphere and its cell culture. Mater. Des. 2016, 104, 60–67. [Google Scholar] [CrossRef]
- Sizeland, K.H.; Hofman, K.A.; Hallett, I.C.; Martin, D.E.; Potgieter, J.; Kirby, N.M.; Hawley, A.; Mudie, S.T.; Ryan, T.M.; Haverkamp, R.G.; et al. Nanostructure of electrospun collagen: Do electrospun collagen fibers form native structures? Materialia 2018, 3, 90–96. [Google Scholar] [CrossRef]
- Berechet, M.D.; Gaidau, C.; Miletic, A.; Pilic, B.; Râpă, M.; Stanca, M.; Ditu, L.-M.; Constantinescu, R.; Lazea-Stoyanova, A. Bioactive properties of nanofibres based on concentrated collagen hydrolysate loaded with thyme and oregano essential oils. Materials 2020, 13, 1618. [Google Scholar] [CrossRef] [Green Version]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Mourya, V.K.; Nazma, N.; Tiwari, I.A. Carboxymethyl chitosan and its applications. Adv. Mat. Lett. 2010, 1, 11–33. [Google Scholar] [CrossRef]
- Shukla, S.K.; Mishra, A.K.; Arotiba, O.A.; Mamba, B.B. Chitosan-Based nanomaterials: A state-of-the-art review. Int. J. Biol. Macromol. 2013, 59, 46–58. [Google Scholar] [CrossRef]
- Chen, Z.; Mo, X.; He, C.; Wang, H. Intermolecular interactions in electrospun collagen–chitosan complex nanofibers. Carbohydr. Polym. 2008, 72, 410–418. [Google Scholar] [CrossRef]
- Inamdar, N.; Mourya, V.K. Chitosan and anionic polymers—Complex formation and applications. In Polysaccharides: Development, Properties and Applications; Tiwari, A., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2010; pp. 333–377. [Google Scholar]
- Chen, Z.G.; Wang, P.W.; Wei, B.; Mo, X.M.; Cui, F.Z. Electrospun collagen–chitosan nanofiber: A biomimetic extracellular matrix for endothelial cell and smooth muscle cell. Acta Biomater. 2010, 6, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Bazmandeh, A.Z.; Mirzaei, E.; Fadaie, M.; Shirian, S.; Ghasemi, Y. Dual spinneret electrospun nanofibrous/gel structure of chitosan-gelatin/chitosan-hyaluronic acid as a wound dressing: In-Vitro and in-vivo studies. Int. J. Biol. Macromol. 2020, 162, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Esparza, Y.; Ullah, A.; Boluk, Y.; Wu, J. Preparation and characterization of thermally crosslinked poly(vinyl alcohol)/feather keratin nanofiber scaffolds. Mater. Des. 2007, 133, 1–9. [Google Scholar] [CrossRef]
- Cardamone, J.M. Investigating the microstructure of keratin extracted from wool: Peptide sequence (MALDI-TOF/TOF) and protein conformation (FTIR). J. Mol. Struct. 2010, 969, 97–105. [Google Scholar] [CrossRef]
- Zhou, Y.; Yao, H.; Wang, J.; Wang, D.; Liu, Q.; Li, Z. Greener synthesis of electrospun collagen/hydroxyapatite composite fibers with an excellent microstructure for bone tissue engineering. Int. J. Nanomed. 2015, 10, 3203–3215. [Google Scholar] [CrossRef] [Green Version]
- Bozec, L.; Odlyha, M. Thermal denaturation studies of collagen by microthermal analysis and atomic force microscopy. Biophys. J. 2011, 101, 228–236. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.P.; Chang, G.Y.; Chen, J.K. Electrospun collagen/chitosan nanofibrous membrane as wound dressing. Colloids Surf. A Physicochem. Eng. Asp. 2008, 313, 183–188. [Google Scholar] [CrossRef]
- Xing, Z.C.; Yuan, J.; Chae, W.P.; Kang, I.K.; Kim, S.Y. Keratin nanofibers as a biomaterial. IPCBEE 2011, 2, 120–124. [Google Scholar]
- Sow, W.T.; Lui, Y.S.; Ng, K.W. Electrospun human keratin matrices as templates for tissue regeneration. Nanomedicine 2013, 8, 531–541. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Li, L.; Mak, A.F.T.; Ko, F.; Qin, L. Preparation and biodegradation of electrospun PLLA/keratin nonwoven fibrous membrane. Polym. Degrad. Stab. 2009, 94, 1800–1807. [Google Scholar] [CrossRef]
- Yuan, J.; Xing, Z.C.; Park, S.W.; Geng, J.; Kang, I.K.; Yuan, J.; Shen, J.; Meng, W.; Shim, K.-J.; Han, I.-S.; et al. Fabrication of PHBV/keratin composite nanofibrous mats for biomedical applications. Macromol. Res. 2009, 17, 850–855. [Google Scholar] [CrossRef]
- Zhou, T.; Mo, X.; Sun, J. Development and properties of electrospun collagen-chitosan nanofibrous membranes as skin wound healing materials. J. Fiber Bioeng. Inform. 2014, 7, 319–325. [Google Scholar]
- Guo, S.; He, L.; Yang, R.; Chen, B.; Xie, X.; Jiang, B.; Weidong, T.; Ding, Y. Enhanced effects of electrospun collagen-chitosan nanofiber membranes on guided bone regeneration. J. Biomater. Sci. Polym. Edit. 2020, 31, 155–168. [Google Scholar] [CrossRef]










| Nanofibers | Flow Rate (mL/h) | Voltage (kV) | Distance from Needle to Collector (cm) |
|---|---|---|---|
| HC10CC/Chitosan | 3.0 | 18.81 | 12 |
| RCG/Acetic acid | 1.4 | 20.53 | 8 |
| KH/PEO | 1.0 | 25.00 | 10 |
| Characteristics | Unit | HC10 | HC10CC | RCG | KH |
|---|---|---|---|---|---|
| Dry substance | % | 9.07 | 57.25 | 11.78 | 9.02 |
| Total ash * | % | 7.72 | 6.57 | 1.61 | 13.73 |
| Total nitrogen * | % | 14.99 | 14.62 | 17.32 | 14.40 |
| Protein substance * | % | 84.24 | 82.16 | 97.28 | 80.84 |
| Aminic nitrogen ** | % | 1.60 | 0.83 | 0.87 | 1.03 |
| Mw | Da | 3,950 | 17,800 | 15,000 | 12,000 |
| pH | pH units | 8.14 | 8.91 | 7.50 | 11.84 |
| Electrical conductivity | μS/cm | 8,400 | 2,360 | 820 | 13,700 |
| Viscosity | cP | 1.5 | 615 | 18,670 | 47 |
| Average particle size | nm | 640.2 | 807 | 858.2 | 1,822 |
| Polydispersity | 0.79 | 0.76 | 0.79 | 0.91 | |
| Zeta potential | mV | −7.28 | −3.89 | −12.2 | −11.4 |
| Sample | Mw (g/mol) | Mn (g/mol) | PDI |
|---|---|---|---|
| HC10CC | 17,124 | 16,075 | 1.06 |
| RCG | 15,479 | 13,509 | 1.15 |
| KH | 13,344 | 7647 | 1.74 |
| Sample | Amide III/(~1454 cm−1) | νAmides I—νAmides II (cm−1) |
|---|---|---|
| HC10CC extract film | 1.06 | 75.5 |
| Electrospun HC10CC | 1.05 | 87.0 |
| RCG extract film | 1.02 | 93.4 |
| Electrospun RCG | 1.01 | 109.5 |
| KH extract film | 0.99 | 88.5 |
| Electrospun KH | 1.01 | 93.7 |
| Sample | α-Helix (%) | β-Strand (%) | β-Turns (%) | Random Coil (%) |
|---|---|---|---|---|
| HC10CC | 17 | 33 | 15 | 35 |
| RCG | 5 | 24 | 14 | 57 |
| KH | 6 | 34 | 1 | 41 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Râpă, M.; Gaidău, C.; Stefan, L.M.; Matei, E.; Niculescu, M.; Berechet, M.D.; Stanca, M.; Tablet, C.; Tudorache, M.; Gavrilă, R.; et al. New Nanofibers Based on Protein By-Products with Bioactive Potential for Tissue Engineering. Materials 2020, 13, 3149. https://doi.org/10.3390/ma13143149
Râpă M, Gaidău C, Stefan LM, Matei E, Niculescu M, Berechet MD, Stanca M, Tablet C, Tudorache M, Gavrilă R, et al. New Nanofibers Based on Protein By-Products with Bioactive Potential for Tissue Engineering. Materials. 2020; 13(14):3149. https://doi.org/10.3390/ma13143149
Chicago/Turabian StyleRâpă, Maria, Carmen Gaidău, Laura Mihaela Stefan, Ecaterina Matei, Mihaela Niculescu, Mariana Daniela Berechet, Maria Stanca, Cristina Tablet, Mădălina Tudorache, Raluca Gavrilă, and et al. 2020. "New Nanofibers Based on Protein By-Products with Bioactive Potential for Tissue Engineering" Materials 13, no. 14: 3149. https://doi.org/10.3390/ma13143149