Removal of Composite Restoration from the Root Surface in the Cervical Region Using Er: YAG Laser and Drill—In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Composite Restoration Procedure
2.3. Composite Removal Techniques
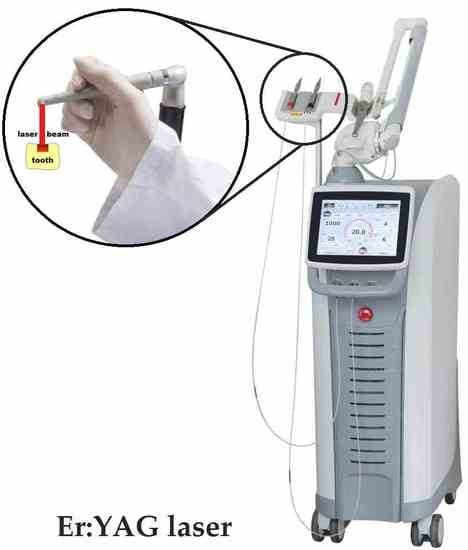
2.3.1. G1 Group (Er: YAG laser)
2.3.2. G2 Group (Dental Bur)
2.4. Fluorescence Microscope Analysis
2.5. Scanning Electron Microscopy
3. Results
3.1. Teeth Surface in a Fluorescence Microscope
3.2. Teeth Surface Analysis in a SEM Microscope
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Verma, S.; Chaudhari, P.; Maheshwari, S.; Singh, R. Laser in dentistry: An innovative tool in modern dental practice. Natl. J. Maxillofac Surg. 2012, 3, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grzech-Leśniak, K.; Nowicka, J.; Pajączkowska, M.; Matys, J.; Szymonowicz, M.; Kuropka, P.; Rybak, Z.; Dobrzyński, M.; Dominiak, M. Effects of Nd:YAG Laser Irradiation on the Growth of Candida Albicans and Streptococcus Mutans: In Vitro Study. Lasers Med. Sci. 2019, 34, 129–137. [Google Scholar] [CrossRef]
- Coluzzi, D.J. Fundamentals of dental lasers: Science and instruments. Dent. Clin. N. Am. 2004, 48, 751–770. [Google Scholar] [CrossRef]
- Matys, J.; Świder, K.; Flieger, R. Laser instant implant impression method: A case presentation. Dent. Med. Probl. 2017, 54, 110–116. [Google Scholar] [CrossRef] [Green Version]
- Perveen, A.; Molardi, C.; Fornaini, C. Applications of laser welding in dentistry: A state-of-the-art review. Micromachines 2018, 9, 209. [Google Scholar] [CrossRef] [Green Version]
- Luke, A.M.; Mathew, S.; Altawash, M.M.; Madan, B.M. Lasers: A review with their applications in oral medicine. J. Lasers Med. Sci. 2019, 10, 324–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matys, J.; Grzech-Leśniak, K.; Flieger, R.; Dominiak, M. Assessment of an impact of a diode laser mode with wavelength of 980 nm on a temperature rise measured by means of k-02 thermocouple: Preliminary results. Dent. Med. Probl. 2016, 53. [Google Scholar] [CrossRef] [Green Version]
- Walsh, L.J. The current status of laser applications in dentistry. Aust. Dent. J. 2003, 48, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Martens, L.C. Laser physics and a review of laser applications in dentistry for children. Eur. Arch. Paediatr. Dent. 2011, 12, 61–67. [Google Scholar] [CrossRef]
- Matys, J.; Hadzik, J.; Dominiak, M. Schneiderian Membrane Perforation Rate and Increase in Bone Temperature During Maxillary Sinus Floor Elevation by Means of Er. Implant Dent. 2017, 26, 238–244. [Google Scholar] [CrossRef]
- Ozaki, M.; Baba, A.; Ishii, K.; Takagi, H.; Motokawa, W. Measurement of bone conduction characteristics for transmitted vibration sounds of tooth drilling. Pediatr. Dent. J. 2007, 17, 148–155. [Google Scholar] [CrossRef]
- Takamori, K.; Furukawa, H.; Morikawa, Y.; Katayama, T.; Watanabe, S. Basic study on vibrations during tooth preparations caused by high-speed drilling and Er:YAG laser irradiation. Lasers Surg. Med. 2003, 32, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Pandurić, D.G.; Bago, I.; Katanec, D.; Žabkar, J.; Miletić, I.; Anić, I. Comparison of Er:YAG Laser and Surgical Drill for Osteotomy in Oral Surgery: An Experimental Study. J. Oral Maxillofac. Surg. 2012, 70, 2515–2521. [Google Scholar] [CrossRef] [PubMed]
- Korkut, E.; Torlak, E.; Gezgin, O.; Özer, H.; Şener, Y. Antibacterial and Smear Layer Removal Efficacy of Er:YAG Laser Irradiation by Photon-Induced Photoacoustic Streaming in Primary Molar Root Canals: A Preliminary Study. Photomed. Laser Surg. 2018, 36, 480–486. [Google Scholar] [CrossRef]
- Yazici, A.R.; Baseren, M.; Gorucu, J. Clinical Comparison of Bur- and Laser-prepared Minimally Invasive Occlusal Resin Composite Restorations: Two-year Follow-up. Oper. Dent. 2010, 35, 500–507. [Google Scholar] [CrossRef]
- Fornaini, C. Er:YAG and adhesion in conservative dentistry: Clinical overview. Laser Ther. 2013, 22, 31–35. [Google Scholar] [CrossRef]
- Zeitouni, J.; Clough, B.; Zeitouni, S.; Saleem, M.; Al Aisami, K.; Gregory, C. The effects of the Er:YAG laser on trabecular bone micro-architecture: Comparison with conventional dental drilling by micro-computed tomographic and histological techniques. F1000Research 2017, 6, 1133. [Google Scholar] [CrossRef] [Green Version]
- DenBesten, P.K.; White, J.M.; Pelino, J.E.P.; Furnish, G.; Silveira, A.; Parkins, F.M. The safety and effectiveness of an Er:YAG laser for caries removal and cavity preparation in children. Med. Laser Appl. 2001, 16, 215–222. [Google Scholar] [CrossRef]
- Sonntag, K.D.; Klitzman, B.; Burkes, E.J.; Hoke, J.; Moshonov, J. Pulpal response to cavity preparation with the Er:YAG and Mark III free electron lasers. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1996, 81, 695–702. [Google Scholar] [CrossRef]
- Featherstone, J.D.B.; Fried, D. Fundamental Interactions of Laserswith Dental Hard Tissues. Med. Laser Appl. 2001, 16, 181–194. [Google Scholar] [CrossRef]
- Zach, L.; Cohen, G. Pulp response to externally applied heat. Oral Surg. Oral Med. Oral Pathol. 1965, 19, 515–530. [Google Scholar] [CrossRef]
- Eriksson, A.R.; Albrektsson, T. Temperature threshold levels for heat-induced bone tissue injury: A vital-microscopic study in the rabbit. J. Prosthet. Dent. 1983, 50, 101–107. [Google Scholar] [CrossRef]
- Apel, C.; Meister, J.; Ioana, R.S.; Franzen, R.; Hering, P.; Gutknecht, N. The Ablation Threshold of Er:YAG and Er:YSGG Laser Radiation in Dental Enamel. Lasers Med. Sci. 2002, 17, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Mirseifinejad, R.; Tabrizizade, M.; Davari, A.; Mehravar, F. Efficacy of Different Root Canal Irrigants on Smear Layer Removal after Post Space Preparation: A Scanning Electron Microscopy Evaluation. Iran. Endod. J. 2017, 12, 185–190. [Google Scholar] [PubMed]
- Almeida, H.C.; Vedovello Filho, M.; Vedovello, S.A.S.; Young, A.A.A.; Ramirez-Yañez, G.O. Er:YAG laser for composite removal after bracket debonding: A qualitative SEM analysis. Int. J. Orthod. Milwaukee. 2009, 20, 9–13. [Google Scholar]
- Dumore, T.; Fried, D. Selective ablation of orthodontic composite by using sub-microsecond IR laser pulses with optical feedback. Lasers Surg. Med. 2000, 27, 103–110. [Google Scholar] [CrossRef]
- Lizarelli, R.D.F.Z.; Moriyama, L.T.; Bagnato, V.S. Ablation of composite resins using Er:YAG laser—Comparison with enamel and dentin. Lasers Surg. Med. 2003, 33, 132–139. [Google Scholar] [CrossRef]
- Kornblit, R.; Trapani, D.; Bossù, M.; Muller-Bolla, M.; Rocca, J.P.; Polimeni, A. The use of Erbium:YAG laser for caries removal in paediatric patients following Minimally Invasive Dentistry concepts. Eur. J. Paediatr. Dent. 2008, 9, 81–87. [Google Scholar]
- Blomlöf, J.P.; Blomlöf, L.B.; Lindskog, S.F. Smear removal and collagen exposure after non-surgical root planing followed by etching with an EDTA gel preparation. J. Periodontol. 1996, 67, 841–845. [Google Scholar] [CrossRef]
- Dilsiz, A.; Aydin, T.; Yavuz, M.S. Root surface biomodification with an Er:YAG laser for the treatment of gingival recession with subepithelial connective tissue grafts. Photomed. Laser Surg. 2010, 28, 511–517. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Cheng, X.; Liu, B.; Liu, X.; Yu, Q.; He, W. Effect of Laser-Activated Irrigations on Smear Layer Removal from the Root Canal Wall. Photomed. Laser Surg. 2017, 35, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Tabrizizadeh, M.; Shareghi, A. The Effect of Preparation Size on Efficacy of Smear Layer Removal; A Scanning Electron Microscopic Study. Iran. Endod. J. 2015, 10, 169–173. [Google Scholar] [PubMed]
- Wahbi, M.A.; Al Sharief, H.S.; Tayeb, H.; Bokhari, A. Minimally invasive use of coloured composite resin in aesthetic restoration of periodontially involved teeth: Case report. Saudi Dent. J. 2013, 25, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Khier, S.; Hassan, K. Efficacy of composite restorative techniques in marginal sealing of extended class v cavities. ISRN Dent. 2011, 2011, 180197. [Google Scholar] [CrossRef] [Green Version]
- Chiniforush, N.; Morshedi, E.; Torabi, S.; Arami, S.; Shahabi, S.; Tabatabaie, M. Assessing microleakage of composite restorations in class V cavities prepared by Er:YAG laser irradiation or diamond bur. J. Conserv. Dent. 2014, 17, 216. [Google Scholar] [CrossRef] [Green Version]
- Weber, M. Handbook of Optical Materials; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Grzech-Leśniak, K.; Matys, J.; Zmuda-Stawowiak, D.; Mroczka, K.; Dominiak, M.; Brugnera, A.; Gruber, R.; Romanos, G.E.; Sculean, A. Er:YAG Laser for Metal and Ceramic Bracket Debonding: An In Vitro Study on Intrapulpal Temperature, SEM, and EDS Analysis. Photomed. Laser Surg. 2018, 36, 595–600. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Kobayashi, K.; Osada, R.; Sakuraba, E.I.; Nomura, T.; Arai, T.; Nakamura, J. Effects of irradiation of an Erbium: YAG laser on root surfaces. J. Periodontol. 1997, 68, 1151–1155. [Google Scholar] [CrossRef]
- Prati, C.; Chersoni, S.; Mongiorgi, R.; Pashley, D.H. Resin-infiltrated dentin layer formation of new bonding systems. Oper. Dent. 1998, 23, 185–194. [Google Scholar]
- Perdigão, J.; Ramos, J.C.; Lambrechts, P. In vitro interfacial relationship between human dentin and one-bottle dental adhesives. Dent. Mater. 1997, 13, 218–227. [Google Scholar] [CrossRef]
- Perdigão, J. Dentin bonding-Variables related to the clinical situation and the substrate treatment. Dent. Mater. 2010, 26. [Google Scholar] [CrossRef]
- Gilpatrick, R.O.; Johnson, W.; Moore, D.; Turner, J. Pulpal response to dentin etched with 10% phosphoric acid. Am. J. Dent. 1996, 9, 125–129. [Google Scholar] [PubMed]
- Gonçalves, L.F.; Fernandes, A.P.; Cosme-Silva, L.; Colombo, F.A.; Martins, N.S.; Oliveira, T.M.; Araujo, T.H.; Sakai, V.T. Effect of EDTA on TGF-β1 released from the dentin matrix and its influence on dental pulp stem cell migration. Braz. Oral Res. 2016, 30, e131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buyukhatipoglu, I.; Secilmis, A. The use of Erbium: Yttrium-aluminum-garnet laser in cavity preparation and surface treatment: 3-year follow-up. Eur. J. Dent. 2015, 9, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Al-Batayneh, O.B.; Seow, W.K.; Walsh, L.J. Assessment of Er:YAG laser for cavity preparation in primary and permanent teeth: A scanning electron microscopy and thermographic study. Pediatr. Dent. 2014, 36, 90–94. [Google Scholar]
- Correa-Afonso, A.M.; Palma-Dibb, R.G.; Pécora, J.D. Composite filling removal with erbium:yttrium-aluminium-garnet laser: Morphological analyses. Lasers Med. Sci. 2010, 25, 1–7. [Google Scholar] [CrossRef]
- Fried, W.A.; Chan, K.H.; Darling, C.L.; Fried, D. Use of a DPSS Er:YAG laser for the selective removal of composite from tooth surfaces. Biomed. Opt. Express 2018, 9, 5026. [Google Scholar] [CrossRef]
- Bertrand, M.F.; Hessleyer, D.; Muller-Bolla, M.; Nammour, S.; Rocca, J.P. Scanning electron microscopic evaluation of resin-dentin interface after Er:YAG laser preparation. Lasers Surg. Med. 2004, 35, 51–57. [Google Scholar] [CrossRef]
- Sasaki, L.H.; Lobo, P.D.C.; Moriyama, Y.; Watanabe, I.-S.; Villaverde, A.B.; Tanaka, C.S.-I.; Moriyama, E.H.; Brugnera, A., Jr. Tensile bond strength and SEM analysis of enamel etched with Er:YAG laser and phosphoric acid: A comparative study In vitro. Braz. Dent. J. 2008, 19, 57–61. [Google Scholar] [CrossRef] [Green Version]
- Matys, J.; Flieger, R.; Dominiak, M. Assessment of Temperature Rise and Time of Alveolar Ridge Splitting by Means of Er:YAG Laser, Piezosurgery, and Surgical Saw: An Ex Vivo Study. Biomed. Res. Int. 2016, 2016, 9654975. [Google Scholar] [CrossRef] [Green Version]
- Berkovitz, B.; Moxham, B.; Linden, R.; Sloan, A. Master Dentistry Volume 3 Oral Biology E-Book: Oral Anatomy, Histology. Available online: https://books.google.pl/books?id=SIcpvee98VAC&pg=PA163&lpg=PA163&dq=The+intertubular+dentin+has+a+more+considerable+amount+of+water+than+the+peritubular+zone&source=bl&ots=MAlmWtbxrN&sig=ACfU3U1AtWbWszv5wqcFGPXqBOMJi8y_Q&hl=en&sa=X&ved=2ahUKEwiSkeW7mq7oAhUFpIsKHYDBDZsQ6AEwCXoECAYQAQ#v=onepage&q=The intertubular dentin has a more considerable amount of water than the peritubular zone&f=false (accessed on 22 March 2020).
- Dilsiz, A.; Aydin, T.; Canakci, V.; Cicek, Y. Root surface biomodification with Nd:YAG laser for the treatment of gingival recession with subepithelial connective tissue grafts. Photomed. Laser Surg. 2010, 28, 337–347. [Google Scholar] [CrossRef] [Green Version]
- Bahrololoomi, Z.; Dadkhah, A.; Alemrajabi, M. The Effect of Er:YAG laser irradiation and different concentrations of sodium hypochlorite on shear bond strength of composite to primary teeth’s dentin. J. Lasers Med. Sci. 2017, 8, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Lahmouzi, J.; Farache, M.; Umana, M.; Compere, P.; Nyssen-Behets, C.; Samir, N. Influence of sodium hypochlorite on Er:YAG Laser-irradiated dentin and its effect on the quality of adaptation of the composite restoration margins. Photomed. Laser Surg. 2012, 30, 655–662. [Google Scholar] [CrossRef] [Green Version]
- Olivi, G.; Olivi, M. Lasers in Restorative Dentistry: A Practical Guide. Available online: https://books.google.pl/books?id=h2B1CgAAQBAJ&pg=PA79&lpg=PA79&dq=sodium+hypochlorite+removal+of+melted+collagen+fiber+after+laser&source=bl&ots=wJgBeyLoDg&sig=ACfU3U1awK3pdxSzm8QMzAUqrCGbWv9t6A&hl=en&sa=X&ved=2ahUKEwiGsd3jm67oAhUj_SoKHXVqDisQ6AEwAHoECAkQAQ#v=onepage&q=sodium hypochlorite removal of melted collagen fiber after laser&f=false (accessed on 22 March 2020).
- Oskoee, P.A.; Oskoee, S.S.; Rikhtegaran, S.; Pournaghi-Azar, F.; Gholizadeh, S.; Aleyasin, Y.; Kasraei, S. Effect of various laser surface treatments on repair shear bond strength of aged silorane-based composite. J. Lasers Med. Sci. 2017, 8, 186–190. [Google Scholar] [CrossRef] [Green Version]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakrzewski, W.; Dobrzynski, M.; Kuropka, P.; Matys, J.; Malecka, M.; Kiryk, J.; Rybak, Z.; Dominiak, M.; Grzech-Lesniak, K.; Wiglusz, K.; et al. Removal of Composite Restoration from the Root Surface in the Cervical Region Using Er: YAG Laser and Drill—In Vitro Study. Materials 2020, 13, 3027. https://doi.org/10.3390/ma13133027
Zakrzewski W, Dobrzynski M, Kuropka P, Matys J, Malecka M, Kiryk J, Rybak Z, Dominiak M, Grzech-Lesniak K, Wiglusz K, et al. Removal of Composite Restoration from the Root Surface in the Cervical Region Using Er: YAG Laser and Drill—In Vitro Study. Materials. 2020; 13(13):3027. https://doi.org/10.3390/ma13133027
Chicago/Turabian StyleZakrzewski, Wojciech, Maciej Dobrzynski, Piotr Kuropka, Jacek Matys, Malgorzata Malecka, Jan Kiryk, Zbigniew Rybak, Marzena Dominiak, Kinga Grzech-Lesniak, Katarzyna Wiglusz, and et al. 2020. "Removal of Composite Restoration from the Root Surface in the Cervical Region Using Er: YAG Laser and Drill—In Vitro Study" Materials 13, no. 13: 3027. https://doi.org/10.3390/ma13133027