Dense Sm and Mn Co-Doped BaTiO3 Ceramics with High Permittivity
Abstract
:1. Introduction
2. Experimental Section
2.1. Sample Preparation
2.2. Characterization
3. Results and Discussion
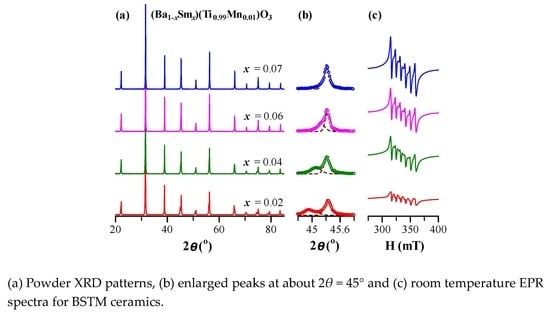
3.1. Structure
3.1.1. SEM Observations
3.1.2. X-ray Diffraction Analysis
3.1.3. Raman Scattering Investigations
3.2. Valence State of Mn Ions
3.3. Dielectric Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Langhammer, H.T.; Müller, T.; Felgner, K.-H.; Abichtm, H.-P. Crystal structure and related properties of manganese-doped barium titanate ceramics. J. Am. Ceram. Soc. 2000, 83, 605–611. [Google Scholar] [CrossRef]
- Jayanthi, S.; Kutty, T.R.N. Dielectric properties of 3d transition metal substituted BaTiO3 ceramics containing the hexagonal phase formation. J. Mater. Sci. Mater. Electron. 2008, 19, 615–626. [Google Scholar] [CrossRef]
- Ueoka, H. The doping effects of transition elements on the PTC anomaly of semiconductive ferroelectric ceramics. Ferroelectrics 1974, 7, 351–353. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, S.-H.; Cho, S.-H. Valence change of Mn in BaTiO3-based PTCR materials. J. Am. Ceram. Soc. 1995, 78, 2845–2848. [Google Scholar] [CrossRef]
- Batllo, F.; Duverger, E.; Jules, J.-C.; Niepce, J.-C.; Jannot, B.; Maglione, M. Dielectric and EPR studies of Mn-doped barium titanate. Ferroelectrics 1990, 109, 113–118. [Google Scholar] [CrossRef]
- Desu, S.B.; Subbarao, E.C. Effect of oxidation states of Mn on the phase stability of Mn-doped BaTiO3. Ferroelectrics 1981, 37, 665–668. [Google Scholar] [CrossRef]
- Rani, A.; Kolte, J.; Gopalan, P. Phase formation, microstructure, electrical and magnetic properties of Mn substituted barium titanate. Ceram. Int. 2015, 41, 14057–14063. [Google Scholar] [CrossRef]
- Lu, D.Y.; Liu, Q.L.; Ogata, T.; Sun, X.Y.; Wang, X.F. Tetragonal phase stabilization caused by Pr ions in Ba(Ti0.99Mn0.01)O3 with mixed phases. Jpn. J. Appl. Phys. 2011, 50, 035806. [Google Scholar] [CrossRef]
- Wang, X.; Gu, M.; Yang, B.; Zhu, S.N.; Cao, W.W. Hall effect and dielectric properties of Mn-doped barium titanate. Microelectron. Eng. 2003, 66, 855–859. [Google Scholar] [CrossRef]
- Glaister, R.M.; Kay, H.F. An investigation of the cubic-hexagonal transition in barium titanate. Proc. Phys. Soc. 1960, 76, 763–771. [Google Scholar] [CrossRef]
- Akishige, Y.; Oomi, G.; Yamamoto, T.; Sawaguchi, E. Dielectric properties of ferroelectric hexagonal BaTiO3. J. Phys. Soc. Jpn. 1989, 58, 930–939. [Google Scholar] [CrossRef]
- Morrison, F.D.; Sinclair, D.C.; West, A.R. Electrical and structural characteristics of lanthanum-doped barium titanate ceramics. J. Appl. Phys. 1999, 86, 6355–6366. [Google Scholar] [CrossRef]
- Yao, Z.H.; Liu, H.X.; Liu, Y.; Wu, Z.H.; Shen, Z.Y.; Liu, Y.; Cao, M.H. Structure and dielectric behavior of Nd-doped BaTiO3 perovskites. Mater. Chem. Phys. 2008, 109, 475–481. [Google Scholar] [CrossRef]
- Park, I.J.; Han, Y.H.; Zhu, K.J.; Wang, J.; Qiu, J.H. Effects of synthesized method on the properties of Sm-doped BaTiO3. Met. Mater. Int. 2014, 20, 1157–1161. [Google Scholar] [CrossRef]
- Sun, Q.M.; Gu, Q.L.; Zhu, K.J.; Wang, J.; Qiu, J.H. Stabilized temperature-dependent dielectric properties of Dy-doped BaTiO3 ceramics derived from sol-hydrothermally synthesized nanopowders. Ceram. Int. 2016, 42, 3170–3176. [Google Scholar] [CrossRef]
- Lu, D.Y.; Toda, M. High-permittivity double rare-earth-doped barium titanate ceramics with diffuse phase transition. J. Am. Ceram. Soc. 2006, 89, 3112–3123. [Google Scholar] [CrossRef]
- Liu, Q.L.; Liu, J.W.; Lu, D.Y.; Zheng, W.T.; Hu, C.Q. Structural evolution and dielectric properties of Nd and Mn co-doped BaTiO3 ceramics. J. Alloy Compd. 2018, 760, 31–41. [Google Scholar] [CrossRef]
- Gong, G.S.; Fang, Y.J.; Zerihun, G.; Yin, C.Y.; Huang, S.; Yuan, S.L. Investigation of A-site La substituted BaTi0.96Mn0.04O3 ceramics: Searching for ferromagnetic origin. J. Appl. Phys. 2014, 115, 243902. [Google Scholar] [CrossRef]
- Dieter, G.E. Mechanical Metallurgy, 3rd ed.; MCgraw-Hill Book Company: New York, NY, USA, 1988; pp. 191–193. [Google Scholar]
- Lu, D.Y.; Yin, S.; Cui, S.Z. A fine-grained and low-loss X8R (Ba1−xDyx)(Ti1−x/2Cax/2)O3 ceramic. J. Alloy Compd. 2018, 762, 282–288. [Google Scholar] [CrossRef]
- Albertsen, K.; Hennings, D.; Steigelmann, O. Donor-acceptor charge complex formation in barium titanate ceramics: Role of firing atmosphere. J. Electroceram. 1998, 2, 193–198. [Google Scholar] [CrossRef]
- Paunovic, V.; Mitic, V.V.; Prijic, Z.; Zivkovic, L. Microstructure and dielectric properties of Dy/Mn doped BaTiO3 ceramics. Ceram. Int. 2014, 40, 4277–4284. [Google Scholar] [CrossRef]
- Kishi, H.; Kohzu, N.; Iguchi, Y.; Sugino, J.; Kato, M.; Ohsato, H.; Okuda, T. Occupation sites and dielectric properties of rare-earth and Mn substituted BaTiO3. J. Eur. Ceram. Soc. 2001, 21, 1643–1647. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Sun, H.; Wang, X.; Yao, X. Structure and electric properties of Sm doped BaTiO3 ceramics. Ferroelectrics 2010, 404, 99–104. [Google Scholar] [CrossRef]
- Tsur, Y.; Dunbar, T.D.; Randall, C.A. Crystal and defect chemistry of rare earth cations in BaTiO3. J. Electroceram. 2001, 7, 25–34. [Google Scholar] [CrossRef]
- Eror, N.G.; Loehr, T.M.; Cornilsen, B.C. Low temperature hexagonal BaTiO3 polymorph and carbonate adsorption. Ferroelectrics 1980, 28, 321–324. [Google Scholar] [CrossRef]
- Venkateswaran, U.D.; Naik, V.M.; Naik, R. High-pressure Raman studies of polycrystalline BaTiO3. Phys. Rev. B 1998, 58, 14256–14260. [Google Scholar] [CrossRef]
- Begg, B.D.; Finnie, K.S.; Vance, E.R. Raman Study of the Relationship between Room-Temperature Tetragonality and the Curie Point of Barium Titanate. J. Am. Ceram. Soc. 1996, 79, 2666–2672. [Google Scholar] [CrossRef]
- Farhi, R.; El Marssi, M.; Simon, A.; Ravez, J. A Raman and dielectric study of ferroelectric Ba(Ti1−xZrx)O3 ceramics. Eur. Phys. J. B 1999, 9, 599–604. [Google Scholar] [CrossRef]
- Dobal, P.S.; Dixit, A.; Katiyar, R.S.; Yu, Z.; Guo, R.; Bhalla, A.S. Phase transition behavior of BaZrxTi1−xO3 ceramics. J. Raman Spectrosc. 2001, 32, 69–71. [Google Scholar] [CrossRef]
- Miao, S.; Pokorny, J.; Pasha, U.M.; Thakur, O.P.; Sinclair, D.C.; Reaney, I.M. Polar order and diffuse scatter in Ba(Ti1−xZrx)O3 ceramics. J. Appl. Phys. 2009, 106, 114111. [Google Scholar] [CrossRef]
- Pokorny, J.; Pasha, U.M.; Ben, L.; Thakur, O.P.; Sinclair, D.C.; Reaney, I.M. Use of Raman spectroscopy to determine the site occupancy of dopants in BaTiO3. J. Appl. Phys. 2011, 109, 114110. [Google Scholar] [CrossRef]
- Feteira, A.; Elsebrock, R.; Dias, A.; Moreira, R.L.; Lanagan, M.T.; Sinclair, D.C. Synthesis and characterisation of La0.4Ba0.6Ti0.6RE0.4O3 (where RE = Y, Yb) ceramics. J. Eur. Ceram. Soc. 2006, 26, 1947–1951. [Google Scholar] [CrossRef]
- Han, D.D.; Lu, D.Y.; Sun, X.Y. Structural evolution and dielectric properties of (Ba1−xNdx)(Ti1−yFey)O3 ceramics. J. Alloys Compd. 2013, 576, 24–29. [Google Scholar] [CrossRef]
- Kchikech, M.; Maglione, M. Electron and lattice excitations in BaTiO3: La. J. Phys. Condens. Matter. 1994, 6, 10159–10170. [Google Scholar] [CrossRef]
- Mazon, T.; Hernandes, A.C.; Filho, A.G.S.; Moraes, A.P.A.; Ayala, A.P.; Freire, P.T.C.; Filho, J.M. Structural and dielectric properties of Nd3+-doped Ba0.77Ca0.23TiO3 ceramics. J. Appl. Phys. 2005, 97, 104113. [Google Scholar] [CrossRef]
- Lu, D.Y.; Cui, S.Z. Defects characterization of Dy-doped BaTiO3 ceramics via electron paramagnetic resonance. J. Eur. Ceram. Soc. 2014, 34, 2217–2227. [Google Scholar] [CrossRef]
- Lu, D.Y.; Cheng, W.; Sun, X.Y.; Liu, Q.L.; Li, D.X.; Li, Z.Y. Abnormal Raman spectra in Er-doped BaTiO3 ceramics. J. Raman Spectrosc. 2014, 45, 963–970. [Google Scholar] [CrossRef]
- Gajović, A.; Tomašić, N.; Djerdj, I.; Su, D.S.; Furić, K. Influence of mechanochemical processing to luminescence properties in Y2O3 powder. J. Alloy. Compd. 2008, 456, 313–319. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, H.; Liu, H.; Zhang, B.B.; Jin, L.; Zhu, M.H.; Yang, W.Q. Theoretical spectra identification and fluorescent properties of reddish orange Sm-doped BaTiO3 phosphors. J. Alloy. Compd. 2015, 643, 247–252. [Google Scholar] [CrossRef]
- Kirianov, A.; Ozaki, N.; Ohsato, H.; Kohzu, N.; Kishi, H. Studies on the solid solution of Mn in BaTiO3. Jpn. J. Appl. Phys. 2001, 40, 5619–5623. [Google Scholar] [CrossRef]
- Kishi, H.; Kohzu, N.; Iguchi, Y.; Sugino, J.; Kato, M.; Ohsato, H.; Okuda, T. Study of occupational sites and dielectric properties of Ho–Mg and Ho–Mn substituted BaTiO3. Jpn. J. Appl. Phys. 2000, 39, 5533–5537. [Google Scholar] [CrossRef]
- Böttcher, R.; Klimm, C.; Michel, D.; Semmelhack, H.-C.; Völkelet, G. Size effect in Mn2+-doped BaTiO3 nanopowders observed by electron paramagnetic resonance. Phys. Rev. B 2000, 62, 2085–2095. [Google Scholar] [CrossRef]
- Ikushima, H.; Hayakawa, S. Electron spin resonance of Mn+2 in BaTiO3. J. Phys. Soc. Jpn. 1964, 19, 1986. [Google Scholar] [CrossRef]
- Wang, X.F.; Liang, P.F.; Chao, X.L.; Yang, Z.P. Dielectric properties and impedance spectroscopy of MnCO3-modified (Ba0.85Ca0.15)(Zr0.1Ti0.9)O3 lead-free ceramics. J. Am. Ceram. Soc. 2015, 98, 1506–1514. [Google Scholar] [CrossRef]
- Cai, W.; Fu, C.L.; Gao, J.C.; Deng, X.L.; Chen, G.; Lin, Z.B. Effect of samarium on the microstructure, dielectric and ferroelectric properties of barium titanate ceramics. Integr. Ferroelectr. 2012, 140, 92–103. [Google Scholar] [CrossRef]








| Composition x | ρb (g/cm3) | ρt (g/cm3) | ρr (%) | GS (μm) |
|---|---|---|---|---|
| 0.02 | 5.61 | 6.02 | 93 | 1.3 |
| 0.04 | 5.62 | 6.04 | 93 | 0.8 |
| 0.06 | 5.65 | 6.06 | 93 | 2.0 |
| 0.07 | 5.62 | 6.08 | 92 | 3.5 |
| Sample | x | ε′m | ε′RT | Tc (°C) | T0 (°C) | C (×105 °C) |
|---|---|---|---|---|---|---|
| BS2TM | 0.02 | 5480 | 2080 | 105.5 | 82.0 | 1.73 |
| BS4TM | 0.04 | 7620 | 3030 | 71.6 | 57.8 | 1.77 |
| BS6TM | 0.06 | 14,252 | 4070 | 39.5 | 40.3 | 1.56 |
| BS7TM | 0.07 | 15,220 | 13,810 | 20.4 | 28.7 | 1.43 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Liu, J.; Lu, D.; Li, T.; Zheng, W. Dense Sm and Mn Co-Doped BaTiO3 Ceramics with High Permittivity. Materials 2019, 12, 678. https://doi.org/10.3390/ma12040678
Liu Q, Liu J, Lu D, Li T, Zheng W. Dense Sm and Mn Co-Doped BaTiO3 Ceramics with High Permittivity. Materials. 2019; 12(4):678. https://doi.org/10.3390/ma12040678
Chicago/Turabian StyleLiu, Qiaoli, Junwei Liu, Dayong Lu, Tingqu Li, and Weitao Zheng. 2019. "Dense Sm and Mn Co-Doped BaTiO3 Ceramics with High Permittivity" Materials 12, no. 4: 678. https://doi.org/10.3390/ma12040678