Comparative Study of Electrophoretic Deposition of Doped BaCeO3-Based Films on La2NiO4+δ and La1.7Ba0.3NiO4+δ Cathode Substrates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of The Electrolyte and Electrode Powders and Their Characterization
2.2. Suspension Preparation and Characterisation
2.3. Electrophoretic Deposition
2.4. Conductivity Measurements
3. Results and Discussion
3.1. Crystal Structure Parameters and Microstructure of The Powders
3.2. Preparation of Stable Suspensions for The EPD
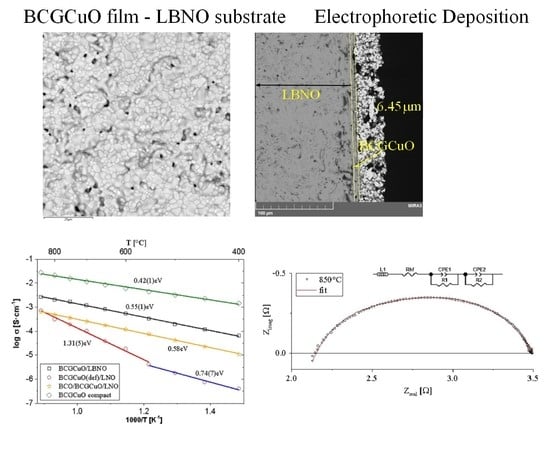
3.3. Electrophoretic Deposition of The Electrolyte Films and Their Characterization
3.4. Electrical Properties of The Electrolyte BCGCuO Film Obtained by Cyclic EPD on the Cathode Substrates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kreuer, K.D. Proton-conducting oxides. Ann. Rev. Mater. Res. 2003, 33, 333–359. [Google Scholar] [CrossRef]
- Medvedev, D.; Murashkina, A.; Pikalova, E.; Demin, A.; Podias, A.; Tsiakaras, P. BaCeO3: Materials development, properties and application. Prog. Mater. Sci. 2014, 60, 72–129. [Google Scholar] [CrossRef]
- Medvedev, D.A.; Lyagaeva, J.G.; Gorbova, E.V.; Demin, A.K.; Tsiakaras, P. Advanced materials for SOFC application: Strategies for the development of highly conductive and stable solid oxide proton electrolytes. Prog. Mater. Sci. 2016, 75, 38–79. [Google Scholar] [CrossRef]
- Matsumoto, H.; Kawasaki, Y.; Ito, N.; Enoki, M.; Ishihara, T. Relation between electrical conductivity and chemical stability of BaCeO3-based proton conductors with different trivalent dopants. Electrochem. Solid St. 2007, 10, B77–B80. [Google Scholar] [CrossRef]
- Hossain, S.; Abdalla, A.M.; Jamain, S.N.B.; Zaini, J.H.; Azad, A.K. A review on proton conducting electrolytes for clean energy and intermediate temperature-solid oxide fuel cells. Renew. Sustain. Energy Rev. 2017, 79, 750–764. [Google Scholar] [CrossRef]
- Ranran, P.; Yan, W.; Lizhai, Y.; Zongqiang, M. Electrochemical properties of intermediate-temperature SOFCs based on proton conducting Sm-doped BaCeO3 electrolyte thin film. Solid State Ionics 2006, 177, 389–393. [Google Scholar] [CrossRef]
- Bae, K.; Jang, D.Y.; Jung, H.J.; Kim, J.W.; Son, J.-W.; Shim, J.H. Micro ceramic fuel cells with multilayered yttrium-doped barium cerate and zirconate thin film electrolytes. J. Power Sources 2014, 248, 1163–1169. [Google Scholar] [CrossRef]
- Dubal, S.U.; Bhosale, C.H.; Jadhav, L.D. Performance of spray deposited Gd-doped barium cerate thin films for proton conducting SOFCs. Ceram. Int. 2015, 41, 5607–5613. [Google Scholar] [CrossRef]
- Zhao, F.; Jin, C.; Yang, C.; Wang, S.; Chen, F. Fabrication and characterization of anode-supported micro-tubular solid oxide fuel cell based on BaZr0.1Ce0.7Y0.1Yb0.1O3−δ electrolyte. J. Power Sources 2011, 196, 688–691. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, Y.-B.; An, J.; Shim, J.H.; Gür, T.M.; Prinz, F.B. Effect of cation non-stoichiometry and crystallinity on the ionic conductivity of atomic layer deposited Y:BaZrO3 films. Thin Solid Films 2013, 539, 166–169. [Google Scholar] [CrossRef]
- Nasani, N.; Ramasamy, D.; Mikhalev, S.; Kovalevsky, A.V.; Fagg, D.P. Fabrication and electrochemical performance of a stable, anode supported thin BaCe0.4Zr0.4Y0.2O3−δ electrolyte protonic ceramic fuel cell. J. Power Sources 2015, 278, 582–589. [Google Scholar] [CrossRef]
- Pikalova, E.; Medvedev, D. Effect of anode gas mixture humidification on the electrochemical performance of the BaCeO3-based protonic ceramic fuel cell. Int. J. Hydrogen Energy 2016, 41, 4016–4025. [Google Scholar] [CrossRef]
- Tarutin, A.; Kasyanova, A.; Lyagaeva, J.; Vdovin, G.; Medvedev, D. Towards high-performance tubular-type protonic ceramic electrolysis cells with all-Ni-based functional electrodes. J. Energy Chem. 2020, 40, 65–74. [Google Scholar] [CrossRef]
- Besra, L.; Liu, M. A review on fundamentals and applications of electrophoretic deposition (EPD). Progr. Mater. Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Corni, I.; Ryan, M.P.; Boccaccini, A.R. Electrophoretic deposition: From traditional ceramics to nanotechnology. J. Eur. Ceram. Soc. 2008, 28, 1353–1367. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Kalinina, E.G. Place of electrophoretic deposition among thin-film methods adapted to the solid oxide fuel cell technology: A short review. Int. J. Energy Prod. Manag. 2019, 4, 1–27. [Google Scholar] [CrossRef]
- Chauoon, S.; Meepho, M.; Chuankrerkkul, N.; Chaianansutcharit, S.; Pornprasertsuk, R. Fabrication of yttria stabilized zirconia thin films on powder-injected anode substrates by electrophoretic deposition technique for solid oxide fuel cell application. Thin Solid Films 2018, 660, 741–748. [Google Scholar] [CrossRef]
- Ichiboshi, H.; Myoujin, K.; Kodera, N.; Ogihara, T. Preparation of Ce0.8Sm0.2O1.9 thin films by electrophoretic deposition and their fuel cell performance. Key Eng. Mater. 2013, 566, 137–140. [Google Scholar] [CrossRef]
- Das, D.; Bagchi, B.; Basu, R.N. Nanostructured zirconia thin film fabricated by electrophoretic deposition technique. J. Alloy. Compd. 2017, 693, 1220–1230. [Google Scholar] [CrossRef]
- Kalinina, E.G.; Efimov, A.A.; Safronov, A.P. The influence of nanoparticle aggregation on the formation of ZrO2 electrolyte thin films by electrophoretic deposition. Thin Solid Films 2016, 612, 66–71. [Google Scholar] [CrossRef]
- Kalinina, E.G.; Pikalova, E.Y.; Menshikova, A.V.; Nikolaenko, I.V. Electrophoretic deposition of a self-stabilizing suspension based on a nanosized multi-component electrolyte powder prepared by the laser evaporation method. Solid State Ionics 2016, 288, 110–114. [Google Scholar] [CrossRef]
- Kalinina, E.G.; Pikalova, E.Y.; Kolchugin, A.A.; Pikalov, S.M.; Kaigorodov, A.S. Cyclic electrophoretic deposition of electrolyte thin-films on the porous cathode substrate utilizing stable suspensions of nanopowders. Solid State Ionics 2017, 302, 126–132. [Google Scholar] [CrossRef]
- Argirusis, C.; Grosse-Brauckmann, J.; Sourkouni, G.; Taillades, G.; Roziere, J. Preparation of thin proton conducting membranes by means of EPD. Key Eng. Mater. 2009, 412, 125–130. [Google Scholar] [CrossRef]
- Bartolomeo, E.D.; Zunic, M.; Chevallier, L.; D’Epifanio, A.; Licoccia, S.; Traversa, E. Fabrication of proton conducting solid oxide fuel cell by using electrophoretic deposition. ECS Trans. 2009, 25, 577–584. [Google Scholar]
- Zunic, M.; Chevallier, L.; Bartolomeo, E.D.; D’Epifanio, A.; Licoccia, S.; Traversa, E. Anode supported protonic solid oxide fuel cells fabricated using electrophoretic deposition. Fuel Cells 2011, 11, 165–171. [Google Scholar] [CrossRef]
- Itagaki, Y.; Yamamoto, Y.; Aono, H.; Yahiro, H. Anode-supported SOFC with thin film of proton-conducting BaCe0.8Y0.2O3−δ by electrophoretic deposition. J. Ceram. Soc. Jpn. 2017, 125, 528–532. [Google Scholar] [CrossRef]
- Kalinina, E.G.; Pikalova, E.Y.; Farlenkov, A.S. Electrophoretic deposition of thin-film coatings of solid electrolyte based on microsize BaCeO3 powders. Russ. J. Appl. Chem. 2018, 91, 934–941. [Google Scholar] [CrossRef]
- Haile, S.M.; Staneff, G.; Ryu, K.H. Non-stoichiometry, grain boundary transport and chemical stability of proton conducting perovskites. J. Mater. Sci. 2001, 36, 1149–1160. [Google Scholar] [CrossRef]
- Mercadelli, E.; Montaleone, D.; Gondolini, A.; Pinasco, P.; Sanson, A. Tape-cast asymmetric membranes for hydrogen separation. Ceram. Int. 2017, 43, 8010–8017. [Google Scholar] [CrossRef]
- Park, I.; Kim, J.; Lee, H.; Park, J.; Shin, D.W. BaCeO3-BaCe0.8Sm0.2O3−δ bi-layer electrolyte-based protonic ceramic fuel cell. Solid State Ionics 2013, 252, 152–156. [Google Scholar] [CrossRef]
- Dubal, S.U.; Jamale, A.P.; Jadhav, S.T.; Patil, S.P.; Bhosale, C.H.; Jadhav, L.D. Yttrium doped BaCeO3 thin films by spray pyrolysis technique for application in solid oxide fuel cell. J. Alloy. Compd. 2014, 587, 664–669. [Google Scholar] [CrossRef]
- Madhuri Sailaja, J.; Murali, N.; Margarette, S.J.; Krishna Jyothi, N.; Rajkumar, K.; Veeraiah, V. Chemically stable proton conducting doped BaCeO3 by citrate-EDTA complexing sol-gel process for solid oxide fuel cell. S. Afr. J. Chem. Eng. 2018, 26, 61–69. [Google Scholar] [CrossRef]
- Ding, Y.; Li, Y.; Huang, W. The role of Ba concentration on the structural characteristics and electrical conductivities of BaxCe0.9Y0.1O3−α. Mater. Res. Bull. 2017, 95, 328–333. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, S.; Li, C.; Rainwater, B.; Liu, Y.; Zhang, L.; Zhang, Y.; Li, C.; Liu, M. Atmospheric plasma-sprayed BaZr0.1Ce0.7Y0.1Yb0.1O3−δ (BZCYYb) electrolyte membranes for intermediate-temperature solid oxide fuel cells. Ceram. Int. 2016, 42, 19231–19236. [Google Scholar] [CrossRef]
- Fabbri, E.; Pergolesi, D.; D’Epifano, A.; Bartolomeo, E.D.; Balestrino, G.; Licoccia, S.; Traversa, E. Design and fabrication of a chemically-stable proton conductor bilayer electrolyte for intermediate temperature solid oxide fuel cells (IT-SOFCs). Energy Environ. Sci. 2008, 1, 355–359. [Google Scholar] [CrossRef]
- Bi, L.; Traversa, E. A chemically stable electrolyte with a novel sandwiched structure for proton-conducting solid oxide fuel cells (SOFCs). Electrochem. Commun. 2013, 36, 42–45. [Google Scholar] [CrossRef]
- Hu, S.; Li, W.; Yao, M.; Li, T.; Liu, X. Electrophoretic deposition of gadolinium-doped ceria as a barrier layer on yttrium-stabilized zirconia electrolyte for solid oxide fuel cells. Fuel Cells 2017, 17, 869–874. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Kolchugin, A.A. The influence of the substituting element (M = Ca, Sr, Ba) in La1.7M0.3NiO4+δ on the electrochemical performance of the composite electrodes. Eur. Chem. Technol. J. 2016, 18, 3–11. [Google Scholar] [CrossRef]
- Gorbova, E.; Maragou, V.; Medvedev, D.; Demin, A.; Tsiakaras, P. Influence of sintering additives of transition metals on the properties of gadolinium-doped barium cerate. Solid State Ionics 2008, 179, 887–890. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Bogdanovich, N.M.; Kolchugin, A.A.; Ananyev, M.V.; Pankratov, A.A. Influence of the synthesis method on the electrochemical properties of bilayer electrodes based on La2NiO4+δ and LaNi0.6Fe0.4O3−δ. Solid State Ionics 2016, 288, 36–42. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Aghajani, H. Suspension characterization and electrophoretic deposition of Yttria-stabilized Zirconia nanoparticles on an iron-nickel based superalloy. Ceram. Int. 2017, 43, 7321–7328. [Google Scholar] [CrossRef]
- Negishi, H.; Yamaji, K.; Sakai, N.; Horita, T.; Yanagishita, H.; Yokokawa, H. Electroforetic deposition of YSZ powders for solid oxide fuel cells. J. Mater. Sci. 2004, 39, 833–838. [Google Scholar] [CrossRef]
- Kalinina, E.G.; Samatov, O.M.; Safronov, A.P. Stable suspensions of doped ceria nanopowders for electrophoretic deposition of coatings for solid oxide fuel cells. Inorg. Mater. 2016, 52, 858–864. [Google Scholar] [CrossRef]
- Kalinina, E.G.; Pikalova, E.Y.; Zhuravlev, V.D.; Scherbinin, S.V.; Safronov, A.P. Aggregatively stable suspensions of micrometer powders of doped barium cerate for electrophoretic deposition of thin-film coatings of solid-oxide fuel cells. Russ. J. Appl. Chem. 2017, 90, 862–869. [Google Scholar] [CrossRef]
- Will, J.; Hruschka, M.K.M.; Gubler, L.; Gauckler, L.J. Electrophoretic deposition of zirconia on porous anodic substrates. J. Am. Ceram. Soc. 2001, 84, 328–332. [Google Scholar] [CrossRef]
- Sora, I.N.; Pelosato, R.; Simone, A.; Montanaro, L.; Maglia, F.; Chiodelli, G. Characterization of LSGM films obtained by electrophoretic deposition (EPD). Solid State Ionics 2006, 177, 1985–1989. [Google Scholar] [CrossRef]
- Krkljuš, I.; Branković, Z.; Duriš, K.; Vukotić, V.; Branković, G.; Bernik, S. The electrophoretic deposition of lanthanum manganite powders for a cathode-supported solid oxide fuel cell in planar and tubular configurations. Int. J. Appl. Ceram. Technol. 2008, 5, 548–556. [Google Scholar] [CrossRef]
- Kalinina, E.G.; Safronov, A.P.; Kotov, Y.A. Formation of thin YSZ electrolyte films by electrophoretic deposition on porous cathodes. Russ. J. Electrochem. 2011, 47, 671–675. [Google Scholar] [CrossRef]
- Aznam, I.; Mah, J.C.W.; Muchtar, A.; Samalu, M.R.; Ghazali, M.J. A review of key parameters for effective electrophoretic deposition in the fabrication of solid oxide fuel cells. J. Zhejiang Univ. Sci. A 2018, 19, 811–823. [Google Scholar] [CrossRef]
- Ishihara, T.; Shimose, K.; Kudo, T.; Nishiguchi, H.; Akbay, T.; Takita, Y. Preparation of yttria-stabilized zirconia thin films on strontium-doped LaMnO3 cathode substrates via electrophoretic deposition for solid oxide fuel cells. J. Am. Ceram. Soc. 2000, 83, 1921–1927. [Google Scholar] [CrossRef]
- Peng, Z.; Liu, M. Preparation of dense platinum-yttria stabilized zirconia and yttria stabilized zirconia films on porous La0.9Sr0.1MnO3 (LSM) substrates. J. Am. Ceram. Soc. 2001, 84, 283–288. [Google Scholar]
- Chen, F.; Liu, M. Preparation of yttria-stabilized zirconia (YSZ) films on La0.85Sr0.15MnO3 (LSM) and LSM-YSZ substrates using an electrophoretic deposition (EPD) process. J. Eur. Ceram. Soc. 2001, 21, 127–134. [Google Scholar] [CrossRef]
- Amsif, M.; Marreno-Lopez, D.; Ruiz-Morales, J.C.; Savvin, S.N.; Gabas, M.; Nunez, P. Influence of rare-earth doping on the microstructure and conductivity of BaCe0.9Ln0.1O3−δ proton conductors. J. Power Sources 2011, 196, 3461–3469. [Google Scholar] [CrossRef]
- Lyagaeva, Y.; Antonov, B.; Dunyushkina, L.; Kuimov, V.; Medvedev, D.; Demin, A.; Tsiakaras, P. Acceptor doping effects on microstructure, thermal and electrical properties of proton-conducting BaCe0.5Zr0.3Ln0.2O3−δ (Ln = Yb, Gd, Sm, Nd, La or Y) ceramics for solid oxide fuel cell applications. Electrochim. Acta 2016, 192, 80–88. [Google Scholar] [CrossRef]
- Danilov, N.; Lyagaeva, J.; Vdovin, G.; Pikalova, E.; Medvedev, D. Electricity/hydrogen conversion by the means of a protonic ceramic electrolysis cell with Nd2NiO4+δ-based oxygen electrode. Energy Convers. Manag. 2018, 172, 129–137. [Google Scholar] [CrossRef]
- Lyagaeva, Y.G.; Danilov, N.A.; Gorshkov, M.Y.; Vdovin, G.K.; Antonov, B.D.; Demin, A.K.; Medvedev, D.A. Functionality of lanthanum, neodymium, and praseodymium nickelates as promising electrode systems for proton-conducting electrolytes. Russ. J. Appl. Chem. 2018, 91, 583–590. [Google Scholar] [CrossRef]
- Medvedev, D.; Lyagaeva, J.; Plaksin, S.; Demin, A.; Tsiakaras, P. Sulfur and carbon tolerance of BaCeO3-BaZrO3 proton-conducting materials. J. Power Sources 2015, 273, 716–723. [Google Scholar] [CrossRef]
- Wang, W.; Medvedev, D.; Shao, Z. Gas humidification impact on the properties and performance of perovskite-type functional materials in proton-conducting solid oxide cells. Adv. Funct. Mater. 2018, 28, 1802592. [Google Scholar] [CrossRef]










| Particles | Processing Method | First Fraction | Second Fraction | ||
|---|---|---|---|---|---|
| Average Size, nm | Content % | Average size, nm | Content % | ||
| BCO | UST 5 min | 152(5) | 13 | 580(17) | 87 |
| UST 25 min | 278(8) | 6 | 1116(33) | 94 | |
| UST 125 min | 197(6) | 4 | 773(23) | 96 | |
| UST 125 min + Centrifugation for 3 min at a speed of 1500 rpm | 41(1) | 12 | 753(23) | 88 | |
| BCGCuO | UST 5 min | 465(14) | 76 | 2473(74) | 24 |
| UST 25 min | 449(13) | 45 | 1384(42) | 55 | |
| UST 125 min | 370(11) | 19 | 1639(49) | 81 | |
| UST 125 min + Centrifugation for 3 min at a speed of 1500 rpm | 60(2) | 19 | 323(10) | 81 | |
| Value, at. % | O | Ni | Cu | Ba | La | Ce | Gd |
|---|---|---|---|---|---|---|---|
| Average | 63 | 0 | 0 | 19 | 1.2 | 16 | 0.7 |
| Standard deviation | 4 | 0 | 0 | 2 | 0.3 | 1.5 | 0.1 |
| Nominal BCGCuO composition | 60 | 0 | 0.2 | 20 | 0 | 17.8 | 2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalinina, E.; Pikalova, E.; Kolchugin, A.; Pikalova, N.; Farlenkov, A. Comparative Study of Electrophoretic Deposition of Doped BaCeO3-Based Films on La2NiO4+δ and La1.7Ba0.3NiO4+δ Cathode Substrates. Materials 2019, 12, 2545. https://doi.org/10.3390/ma12162545
Kalinina E, Pikalova E, Kolchugin A, Pikalova N, Farlenkov A. Comparative Study of Electrophoretic Deposition of Doped BaCeO3-Based Films on La2NiO4+δ and La1.7Ba0.3NiO4+δ Cathode Substrates. Materials. 2019; 12(16):2545. https://doi.org/10.3390/ma12162545
Chicago/Turabian StyleKalinina, Elena, Elena Pikalova, Alexandr Kolchugin, Nadezhda Pikalova, and Andrey Farlenkov. 2019. "Comparative Study of Electrophoretic Deposition of Doped BaCeO3-Based Films on La2NiO4+δ and La1.7Ba0.3NiO4+δ Cathode Substrates" Materials 12, no. 16: 2545. https://doi.org/10.3390/ma12162545