3.1. Synthesis of Boron Nitride Nanomaterials
For some metals, formation enthalpies with boron (H
forB) and nitrogen (H
forN) are indicated in
Figure 1a,b, respectively. The data were from theoretical calculations [
8]. Difference of formation enthalpy (H
forN-H
forB) is also shown in
Figure 1c. The difference of formation enthalpy (H
forN-H
forB) is very important for the formation of BN fullerene nanomaterials, because reactivity with nitrogen and boron is decided by this enthalpy. Basically, BN nanotubes are formed when rare earth metals are used as catalytic metals, such as Y, Zr, Nb, Hf, Ta, W and La. These elements have minus enthalpy, as shown in
Figure 1c. It means that catalytic elements for synthesis of BN nanotubes should be selected from those with minus formation enthalpy (H
forN-H
forB). From the present guideline, Sc element could be a good catalytic element to form BN nanotubes. The detailed formation mechanism of the nanostructures with different metals should be investigated further.
A HREM image of a BN nanotube produced using LaB
6 powder is shown in
Figure 2a. In
Figure 2a, the diameter of the five-layered BN nanotube is changing from bottom to top, and amorphous patches are observed mostly at the top. Low magnification images of BN nanostructures were reported [
17,
20]. A HREM image of BN nanocage produced from LaB
6/B powder is shown in
Figure 2b, which indicates square-like shape, and four-membered rings of BN exist at the corner of the cage. The BN nanocage has a network-like structure, whose atomic arrangement is basically consistent with the B
36N
36 cluster structure [
8]. BN nanocapsules with Pd nanoparticles were also produced as shown in
Figure 2c, and Pd nanoparticles were covered by a few BN layers. In order to confirm the formation of BN nanocapsules, EDX analysis was carried out, which showed the atomic ratio of B:N ≈ 1.
Figure 1.
(a) Formation enthalpy with boron (HforB) and (b) nitrogen (HforN); (c) difference of formation enthalpy (HforN-HforB).
Figure 1.
(a) Formation enthalpy with boron (HforB) and (b) nitrogen (HforN); (c) difference of formation enthalpy (HforN-HforB).
Figure 2.
High-resolution electron microscopy (HREM) images of BN nanotube and BN nanocapsules produced using powder with ratios of: (a) La:B = 1:6; (b) La:B = 1:4; and (c) Pd:B = 1:4.
Figure 2.
High-resolution electron microscopy (HREM) images of BN nanotube and BN nanocapsules produced using powder with ratios of: (a) La:B = 1:6; (b) La:B = 1:4; and (c) Pd:B = 1:4.
3.2. Hydrogen Storage in Boron Nitride Nanomaterials Studied by Thermogravimetry/Differential Thermogravimetric Analysis
DTA and TG curve of BN nanomaterials produced from LaB
6 powder is shown in
Figure 3. At a temperature around 70 °C, an increase of sample weight of 0.3 mg is observed. Weight change for this sample was almost reversible, which indicates the reversibility of hydrogen adsorption. It also suggests that the hydrogen atoms would be physically absorbed. For the samples of La:B = 1:6 and Pd:B = 1:4, weight increases of 3.2% and 1.6% were observed, respectively, as listed in
Table 2.
Figure 3.
Differential thermogravimetric analysis (DTA) and thermogravimetry (TG) curve of BN nanocapsules and nanotubes produced using LaB6 powder.
Figure 3.
Differential thermogravimetric analysis (DTA) and thermogravimetry (TG) curve of BN nanocapsules and nanotubes produced using LaB6 powder.
Table 2.
Atomic ratio of starting materials, produced structures and hydrogen storage.
Table 2.
Atomic ratio of starting materials, produced structures and hydrogen storage.
| M:B | Nanostructures | Weight change (wt%) |
|---|
| La:B = 1:6 | Nanotubes and nanocages | +3.2 |
| La:B = 1:10 | Nanotubes | +0.58 |
| Pd:B = 1:4 | Nanocapsules | +1.6 |
BN nanostructures would have energy barriers for H
2 molecules to pass through tetragonal and hexagonal rings.
Figure 4 are structure models in which H
2 molecule passes from hexagonal rings of B
99N
99 and B
36N
36. Single point energies were calculated with changing set point of H
2 molecule from the center of the cage at intervals of 0.1 nm. There is energy barrier that is given for H
2 molecules to pass through the hexagonal rings. The energy barrier of B
36N
36 hexagonal rings showed the smallest value of 14 eV in the present calculation, which is smaller than that of 27 eV at tetragonal rings. The DE of C
60 hexagonal rings was also calculated to be 16 eV for comparison. This value is higher than that of B
36N
36 hexagonal rings, and the H
2 molecule would pass from hexagonal rings of B
36N
36 easier than from hexagonal rings of C
60. It is known that H
2 molecules are adsorbed on walls of single-walled carbon nanotubes over 7 MPa as an experimental result. As a result of the comparison, H
2 molecules enter into B
36N
36 from hexagonal rings easier than tetragonal rings B
36N
36 and hexagonal rings of C
60.
Figure 4.
Structure models that H2 molecule passes through hexagonal BN rings of (a,b) tip of B99N99 nanotube and (c) B36N36 cluster.
Figure 4.
Structure models that H2 molecule passes through hexagonal BN rings of (a,b) tip of B99N99 nanotube and (c) B36N36 cluster.
A formation mechanism of BN nanotubes and nanocapsules synthesized in the present work is described below. Metal and boron particles are melted by arc-melting, and during the solidification of the liquid into metal and/or boride nanoparticles, excess boron would react with nitrogen to form BN layers at the surface of the nanoparticles. Because of electrical insulation, BN fullerene materials are usually fabricated by arc-discharge method with specific conducting electrodes such as HfB2 and ZrB2. The present arc-melting method from mixed powder has two advantages for BN nanomaterial production. Since the powder becomes conducting by pressing, special electrodes are not needed. In addition, ordinary, commercial arc-melting furnaces can be used. These advantages indicate a simpler fabrication method compared to the ordinary arc-discharge methods.
Although gas storages of hydrogen and argon in carbon nanotubes have been reported, there are few reports for gas storage in BN fullerene materials and for calculations [
21]. Weight increase of the sample in TG measurements was observed as shown in
Figure 3. It might be due to the hydrogen gas storage in the BN nanomaterials. Since there would be metal and boron nanoparticles in the separated BN nanomaterials even after the separation, further qualification and evaluation of the samples are needed for hydrogen storage.
Carbon fullerenes and boron nitride fullerenes are sublimed at 600 and 1000 °C, respectively. Boron nitride fullerenes would storage H2 molecules with smaller energy than carbon fullerenes, and would give good stability at high temperature. Boron nitride fullerene materials would be a better candidate for H2 storage materials.
3.3. Molecular Orbital Calculations of Hydrogen Storage in Boron Nitride and C Clusters
Figure 5 is a structural model of hydrogen atoms chemisorbed on boron and nitrogen for BN clusters and carbon clusters. Atoms bonded with hydrogen are moved outside from the clusters. Energies for hydrogen chemisorption on each position are summarized as
Table 3. Hydrogen bonding with nitrogen is more stable than that with boron, and hydrogen bonding with carbon is more stable than C
60. This result indicates the chemical modification of carbon fullerenes. When two hydrogen atoms were chemisorbed on carbon clusters, energies of carbon clusters increased.
Figure 5.
Structural models of H atoms chemisorbed on (a) C60 and (b) B24N24.
Figure 5.
Structural models of H atoms chemisorbed on (a) C60 and (b) B24N24.
Table 3.
Chemisorption energy of hydrogen (H) atoms on C60 and B24N24. ΔE = (Heat of formation after hydrogen addition) − (Heat of formation before hydrogen addition).
Table 3.
Chemisorption energy of hydrogen (H) atoms on C60 and B24N24. ΔE = (Heat of formation after hydrogen addition) − (Heat of formation before hydrogen addition).
| Cluster | Number of H atoms | Additional position of H | Heat of formation (eV) | ΔE (eV) |
|---|
| Before addition | After addition |
|---|
| C60 | 1 | C | 35.21 | 35.03 | −0.18 |
| 2 | C | 35.21 | 35.81 | 0.6 |
| B24N24 | 1 | B | −36.12 | −34.66 | 1.46 |
| 1 | N | −36.12 | −35.67 | 0.45 |
| B36N36 | 1 | B of tetragonal ring | −69.33 | −67.83 | 1.50 |
| 1 | N of tetragonal ring | −69.33 | −69.16 | 0.17 |
| 1 | B of tetragonal ring | −69.33 | −67.51 | 1.83 |
| 1 | N of tetragonal ring | −69.33 | −68.83 | 0.51 |
In the calculation, chemisorption of hydrogen was performed on outside of cage, and on boron, nitrogen and carbon of tetragonal and hexagonal rings. The B
36N
36 cluster has tetragonal and hexagonal BN rings, and there are four kinds of boron and nitrogen positions for the hydrogen chemisorption, as shown in
Figure 6. For the stability calculations of H
2 molecules in these clusters, 30 H
2 molecules were also introduced in B
24N
24, B
36N
36, B
60N
60 and C
60.
Figure 5 is a structural model of hydrogen atoms chemisorbed on boron and nitrogen for B
36N
36. Energies for hydrogen chemisorption on each position are summarized as
Table 3. Hydrogen bonding with nitrogen is more stable than that with boron because nitrogen atoms are more electrophilic compared to boron atoms. In addition, hydrogen bonding on tetragonal ring is more stable than that of hexagonal ring. Chemisoption of hydrogen with C
60 reduced the energy. When two hydrogen atoms were chemisorbed on carbon clusters, energies of carbon clusters increased.
Figure 6.
Structure models for hydrogen chemisorption on B36N36: (a) structural model of B36N36; (b,c) structural models of hydrogen chemisorption on N and B positions of tetragonal BN ring, respectively; and (d,e) structural models of hydrogen chemisorbed on N and B positions of hexagonal BN ring, respectively.
Figure 6.
Structure models for hydrogen chemisorption on B36N36: (a) structural model of B36N36; (b,c) structural models of hydrogen chemisorption on N and B positions of tetragonal BN ring, respectively; and (d,e) structural models of hydrogen chemisorbed on N and B positions of hexagonal BN ring, respectively.
To investigate stability of H
2 molecules in clusters, energies were calculated for H
2 molecules introduced in the center of clusters. The structural models are shown in
Figure 7. Energies of C
60, B
24N
24, B
36N
36 and B
60N
60 were calculated to be 0.58, 20.71, 20.93 and 20.82 eV/mol atom, respectively. This result indicates that B
24N
24, B
36N
36 and B
60N
60 with H
2 molecules are more stable than C
60 with H
2 molecule, and that B
36N
36 is the most stable in BN clusters.
Figure 7.
Optimized structural models of H2 molecules in the clusters. A H2 molecule in the center of (a) C60; (b) B24N24; (c) B36N36; (d) 22 H2 molecules inside C60 and 8 atoms chemisorbed; (e) 9 H2 molecules in B24N24; and (f) 20 molecules in B36N36; (g) 1 and (h) 38 H2 molecules in B60N60.
Figure 7.
Optimized structural models of H2 molecules in the clusters. A H2 molecule in the center of (a) C60; (b) B24N24; (c) B36N36; (d) 22 H2 molecules inside C60 and 8 atoms chemisorbed; (e) 9 H2 molecules in B24N24; and (f) 20 molecules in B36N36; (g) 1 and (h) 38 H2 molecules in B60N60.
Hydrogen storage (wt%) of B
60N
60 cluster was calculated, and structural models are shown in
Figure 7. Other C
60, B
24N
24 and B
36N
36 were calculated, as summarized in
Table 4. From
Table 4, C and BN cluster showed possibility of hydrogen storage of ~6.5 and ~4.9 wt%, respectively.
Although hydrogen storage (wt%) of C
60 is better than those of BN clusters, energy increase by hydrogen addition is higher for C
60 (4.6–5.2 eV/H atom) compared to BN clusters (1.0–3.1 eV/H atom), which would be due to C–H interaction. This indicates that needed energy for hydrogen storage in BN clusters is lower compared to the C
60. From
Table 4, stability of H
2 molecules in B
24N
24 and B
36N
36 seems to be higher than that of C
60. Hydrogen atoms were calculated to be chemisorbed inside C
60, as shown in
Table 4. This indicates that inside of C
60 also has good reactivity as well as outside of the cage. Chemisorption inside the C
60 clusters may indicate that hydrogen capacity may gradually reduce under the adsorption-desorption cycles. On the other hand, BN clusters have no chemisorption inside the clusters, which indicates that the BN clusters would be a better candidate for stable adsorption-desorption cycles. C
60 cluster shows the minimum energies in spite of the positive values. It is believed that p-electrons outside and inside of the cage would increase the energy. However, s-electrons in the 5- and 6-membered carbon rings would stabilize the cage structure. Hydrogen addition to the C
60 decreased the energy, which agrees with the above description. The hydrogen storage of the present BN cluster was calculated to be ~4.9 wt%, and the measured values were 1–3 wt%. This might be due to the remained catalytic metals in the sample, which reduces the storage ratio.
Table 4.
Energy of clusters with hydrogen.
Table 4.
Energy of clusters with hydrogen.
| Cluster | Introduced H2 | Heat of formation (eV) | H atoms chemisorbed inside cluster | Hydrogen storage (wt%) | Heat of formation per added H atom (eV/H atom) |
|---|
| C60 | 0 | 35.21 | 0 | 5.8–6.5 | 4.9–5.2 |
| 22 | 143.01 | 0 |
| 25 | 164.87 | 4 |
| 26 | 169.63 | 8 * |
| B24N24 | 0 | −36.12 | 0 | 2.9 | 3.0 |
| 9 | −9.44 | 0 |
| B36N36 | 0 | −69.33 | 0 | 4.3 | 3.1 |
| 20 | −6.66 | 0 |
| B60N60 | 0 | −100.28 | 0 | 4.9 | 1.0 |
| 38 | −61.74 | 0 |
Although carbon nanotubes are oxidized at ~600 °C, BN nanomaterials are almost stable ~900 °C in air, which indicates BN fullerenes have higher thermal and chemical stability than those of carbon fullerenes. BN fullerenes with good thermal and chemical stability can store H2 molecules with less energy, and they have the same chemisorption energy and higher stability, compared to carbon clusters. BN fullerene materials would be better candidates for H2 storage materials.
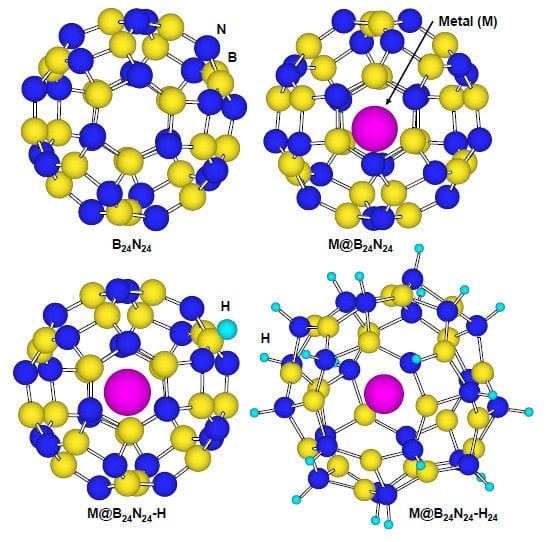
3.4. Effects of Endohedral Element in B24N24 Clusters on Hydrogenation
Figure 8 is a structural model of hydrogen atoms chemisorbed on nitrogen position for M@B
24N
24. Energies for hydrogen chemisorption on boron and nitrogen positions are summarized as
Table 5. Heats of formation (eV) of M@B
24N
24 by hydrogenation are indicated in
Figure 9. In
Table 5 and
Figure 9, “None” means that metal catalyst was not encaged in B
24N
24, and “BH” and “NH” means that hydrogen atom was chemisorbed on boron and nitrogen, respectively.
Figure 9 shows that hydrogen bonding with nitrogen is more stable than that with boron because nitrogen atoms are more electrophilic compared to boron atoms.
Figure 9 also indicates that energies of chemisorption on M@B
24N
24 are much lower than that of B
24N
24. From this result, Li atom works as a good endohedral element for hydrogen chemisorption. Metal catalysts in the present work have been reported to generate hydrides such as LiH because of strong interaction between hydrogen and metal atoms. In the present work, it is clarified that Li doping and nitrogen positions are suitable for hydrogenation for the B
24N
24 clusters.
Figure 8.
Structural models for (a) B24N24; (b) endohedral M@B24N24; (c) hydrogenated M@B24N24-H; and (d) M@B24N24 which chemisorbed 24 hydrogen atoms.
Figure 8.
Structural models for (a) B24N24; (b) endohedral M@B24N24; (c) hydrogenated M@B24N24-H; and (d) M@B24N24 which chemisorbed 24 hydrogen atoms.
Table 5.
Chemisorption energies of a hydrogen atom on M@B24N24.
Table 5.
Chemisorption energies of a hydrogen atom on M@B24N24.
| Cluster | Hydrogenation position | Heat of formation (eV) | ΔE * (eV) |
|---|
| B24N24 | Un-hydrogenated | −36.12 | 0 |
| B | −34.66 | 1.46 |
| N | −35.67 | 0.45 |
| Li@B24N24 | Un-hydrogenated | −36.79 | 0 |
| B | −37.96 | −1.17 |
| N | −38.05 | −1.26 |
| Na@B24N24 | Un-hydrogenated | −35.38 | 0 |
| B | −36.43 | −1.05 |
| N | −36.62 | −1.24 |
| K@B24N24 | Un-hydrogenated | −31.80 | 0 |
| B | −32.84 | −1.04 |
| N | −32.90 | −1.10 |
Figure 9.
Heats of formation of hydrogenation for M@B24N24 clusters by endohedral elements.
Figure 9.
Heats of formation of hydrogenation for M@B24N24 clusters by endohedral elements.
Bond lengths of B–H and N–H for M@B
24N
24 are summarized in
Table 6. B–H and N–H distance decreased by doping element in the BN cluster. Bond lengths of N–H are shorter than that of B–H for these BN clusters, and the bond length of N–H for Li@B
24N
24 is the shortest. The endohedral atoms appear to decrease the repulsion energy between the electrons of the hydrogen atom and the p-electrons of B
24N
24.
Figure 8d is a structural model of M@B
24N
24H
24, which indicates hydrogenation on all nitrogen positions for M@B
24N
24, and the hydrogen storage capacity is summarized in
Table 7. Li is also a good element for hydrogen storage capacity because Li is the lightest element of these. Energy levels and density of states for B
24N
24 and Li@B
24N
24 are shown in
Figure 10. Fermi levels in energy level diagrams and DOS diagrams correspond to 0 eV. B
24N
24 and Li@B
24N
24 show energy gaps of 4.8873 and 0.0247 eV between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO), respectively. This means that electron of Li element transferred to the B
24N
24 cage, and electronic state of BN cluster would change from semiconductor to metallic property by Li doping in BN clusters.
Table 6.
Bond length of B–H and N–H on M@B24N24.
Table 6.
Bond length of B–H and N–H on M@B24N24.
| Cluster | B–H (Å) | N–H (Å) |
|---|
| B24N24 | 1.229 | 1.010 |
| Li@B24N24 | 0.695 | 0.616 |
| Na@B24N24 | 1.203 | 1.005 |
| K@B24N24 | 1.204 | 1.006 |
Table 7.
Hydrogen storage capacity of chemisorption on nitrogen position of M@B24N24.
Table 7.
Hydrogen storage capacity of chemisorption on nitrogen position of M@B24N24.
| Cluster | Hydrogen storage (wt%) |
|---|
| Li@B24N24H24 | 3.86 |
| Na@B24N24H24 | 3.76 |
| K@B24N24H24 | 3.67 |
Figure 10.
Energy levels diagrams and density of states of (a) B24N24 and (b) Li@B24N24.
Figure 10.
Energy levels diagrams and density of states of (a) B24N24 and (b) Li@B24N24.
Although it was reported that BN nanotubes were produced by lithium vapor, synthesis of Li@BN has not been reported yet. BN fullerenes have high thermal and chemical stability, and M@BN fullerenes have lower energy of chemisorption compared to the present work. Since the BN clusters were reported to be doped with metal elements, M@BN clusters would be produced. BN fullerene materials with endohedral element such as Li would be better candidates for H2 storage materials.
3.5. Molecular Dynamics Calculations of Hydrogen Storage in C60 Clusters
C
60 included a H
2 molecule kept in stable state at
T = 298 K and
P = 0.1 MPa. This unit cell is shown in
Figure 11. This model was calculated with
N = 62 atoms,
T = 298 K and
P = 0.1 MPa. Although the H
2 molecule vibrated in the C
60 cage, it was not discharged from the cage. Some H
2 molecules were stored in the C
60 cage when the pressure was 5 MPa. This unit cell is shown in
Figure 12, which is composed of 32 C
60 (
fcc) with 288 H
2 molecules (
fcc). This model was calculated with
N = 2496 atoms and
P = 5 MPa. H
2 molecules pass through the hexagonal rings of the C
60 cage at 0.5 ps.
Figure 11.
Molecular dynamics calculation to confirm stability of H2 molecule into C60 at 298 K and 105 Pa. NTP ensembles and organic potential were used in the calculation.
Figure 11.
Molecular dynamics calculation to confirm stability of H2 molecule into C60 at 298 K and 105 Pa. NTP ensembles and organic potential were used in the calculation.
Figure 12.
Molecular dynamics calculation to find condition of H2 gas storage. Unit cell is composed from 32 C60 (fcc) with 288 H2 molecules (fcc). NPH ensembles and organic potential were used in the calculation.
Figure 12.
Molecular dynamics calculation to find condition of H2 gas storage. Unit cell is composed from 32 C60 (fcc) with 288 H2 molecules (fcc). NPH ensembles and organic potential were used in the calculation.
After introducing H
2 molecules into the C
60 cage at 2.5 ps, they are stored and stable in C
60.
Figure 13 shows a schematic model of a single H
2 molecule stored in a hexagonal ring of C
60. These results indicate that fullerene-like materials can store H
2 gas in cage at
T = 298 K and
P = 0.1 MPa, and some H
2 molecules were stored in the C
60 cage when the pressure was greater than 5 MPa. It is known that H
2 molecules are adsorbed on the walls of single-walled carbon nanotubes over 7 MPa as an experimental result [
26]. Since a C
60 cluster has a large curvature and H
2 molecules are very small compared to C
60, it is considered that adsorption and storage of H
2 gas occur at the same time. Detailed theoretical calculation on hydrogen storage in BN nanomaterials has also been performed [
27,
28,
29,
30,
31].
Figure 13.
Schematic model of H2 molecule stored in hexagonal ring in the order of (a–d).
Figure 13.
Schematic model of H2 molecule stored in hexagonal ring in the order of (a–d).